Turning back the clock of neural progenitor cells: a simple recipe to generate de novo retinal ganglion cells
Posted by the Node, on 12 August 2019
Press release for a new Development paper on reprogramming in the retina.
Scientists at the Federal University of Rio de Janeiro, Brazil, in collaboration with the Max Planck Institute of Molecular Cell Biology and Genetics, Germany, discovered that a single transcription factor drives retinal progenitor cells to reacquire the potency to generate Retinal ganglion cells. The transcription factor is Klf4, which became notorious for its role in reprogramming cells towards pluripotency. The novel findings open a new door to investigate regenerative therapies for retinal diseases that cause irreversible vision loss, such as Glaucoma. The research was published in the scientific journal Development at https://dev.biologists.org/content/early/2019/08/08/dev.176586.
Ganglion cells in the retina are the source of the optic nerve and are vital for seeing as this nerve sends the messages from the retina to the brain. Their degeneration in glaucoma leads millions of people to permanent vision impairment. Current treatments at best control the progression of the disease, but recovery of normal vision require not only the prevention of cell death but also regeneration of cells and their axons.
To address the latter, Mauricio Rocha-Martins, Mariana Silveira and a team of researchers investigated both the role of Klf4 in the normal development of ganglion cells, as well as its potential to coax retinal progenitor cells to generate these neurons.
Although an endogenous role of Klf4 in retinal development could not be confirmed, overexpression of this gene in retinal progenitors of newborn rats led to a switch in cell fate. Instead of giving birth mostly to photoreceptors (which sense light) the progenitors generated retinal ganglion cells, the projection neurons of the retina. Remarkably, less than two days after Klf4 overexpression, a molecular program was activated that is similar to that responsible for the generation of ganglion cells during retinal development.
“We literally cheered the first time we noticed that, instead of positioning on the photoreceptor layer, where most of those cells should be in normal conditions, this transcription factor led the progeny of progenitor cells to move to the region of the retinal ganglion cells, and project axons toward the optic nerve,” the authors said.
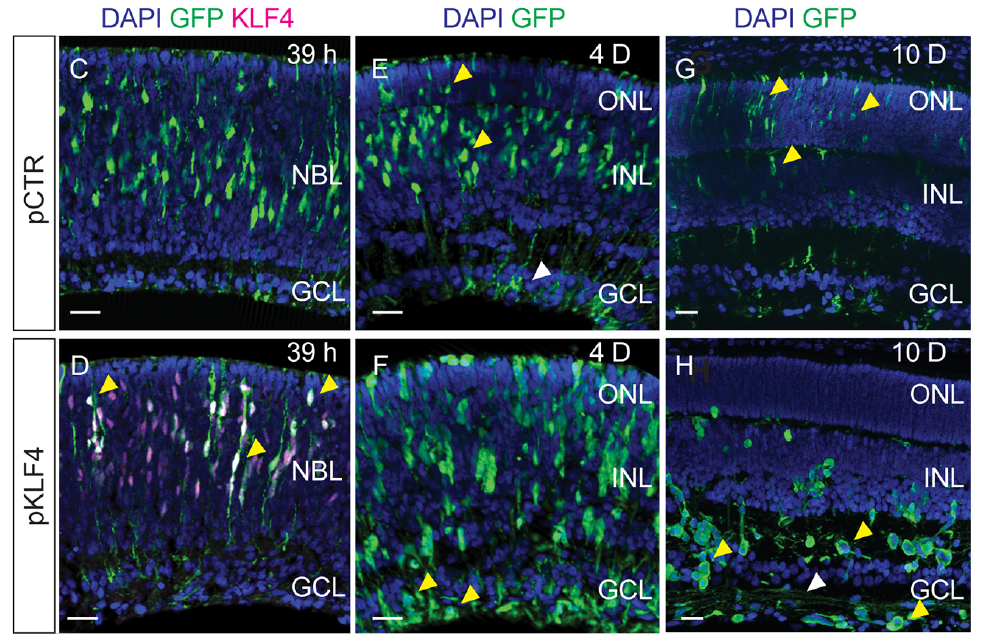
Future work will focus both on improving the protocol to guarantee that these induced ganglion cells acquire mature properties, but also to direct this strategy to the glial cells of the retina, of which the ability to regenerate retinal ganglion cells was lost in mammals through evolution, but remains in fish.
“We believe there is a long way towards actual therapy, and plenty to be understood, but our data indicate that the program to generate ganglion cells can be reactivated, which may open new directions for regenerative therapies,” said the authors. “This is a starting point,” they conclude.


 (5 votes)
(5 votes)