Uncovering the origins of the adult adipose tissue in flies
Posted by Tadashi Uemura, on 24 May 2023
Dr Taiichi Tsuyama, Professor Tadashi Uemura and colleagues from Kyoto University recently published a paper in Development entitled ‘Dynamic de novo adipose tissue development during metamorphosis in Drosophila melanogaster‘, identifying the precursor cells that give rise to the adult fat body in Drosophila. We caught up with the authors to learn more about the story behind this work.
What were known about the origin and developmental processes of adult adipose tissue in fruit flies before your work?
At the start of this project, many fly people knew that the adult fat body (AFB) exists in adult flies immediately after eclosion; however, only several studies tried to reveal the developmental aspects of the AFB in detail. Using transplantation techniques, Lawrence and Johnston (1986) reported that the AFB is mesodermal in embryonic origin. Hoshizaki et al. (1995) tackled the origin of the AFB using histochemical techniques with state-of-the-art genetic reporter lines in those days. The Hoshizaki paper has been one of the best references for the development of the AFB for about 30 years. However, it had been cited only ~30 times by 2021 despite many studies employing the mature AFB to study fat metabolism in adult flies. No previous study had identified precursor cells of AFB and characterized their cellular dynamics underlying AFB formation.
Why is this such a challenge to unravel?
We think a major obstacle was the lack of genetic tools that specifically control gene expression in the AFB but not in the larval fat body (LFB). The larval fat body cells, which are generated in the embryo, persist during metamorphosis and locate near the AFB in young adult flies. Thus, genetic tools specific to the AFB are required to unravel the developmental progress of the AFB. Our interest in the adult fat body might be kind of serendipitous. One central theme in our laboratory has been how neuronal dendritic arbors achieve their complex and diverse morphological patterns and how they undergo remodeling during metamorphosis (for example, Shimono et al. 2014; Tsuyama et al. 2017). When we had attempted to study how systemic communications affect the metamorphic remodeling of dendritic trees in flies, we noticed that there were no good tools to control gene expression in larval and adult fat cells individually during metamorphosis. It prompted us to establish new genetic tools, which enabled us to visualize the developmental progression of the AFB in metamorphic flies.
Can you summarise your key findings?
We identified precursor cells that give rise to the AFB and delineated their dynamic cellular behaviors at the single-cell resolution (Figure 1; Tsuyama et al. 2023). These precursor cells emigrate from the thorax with polarized cell shapes and oriented motility, and undergo a long journey to disperse to the abdomen and head. After this spatiotemporal large-scale migration, these cells adhere to each other, assembling into the AFB with a sheet-like architecture. Cell proliferation takes place continuously during and after the migration to make up one of the largest tissues in the abdomen of adult flies. Another intriguing behavior is homotypic cell fusion after the sheet formation, resulting in the formation of multinucleated adult fat cells. We also tested the roles of candidate genes and found that Ecdysone Receptor (EcR), a steroid hormone receptor critical for the metamorphic progression of insects, and the GATA-factor transcription factor Serpent support AFB organogenesis.
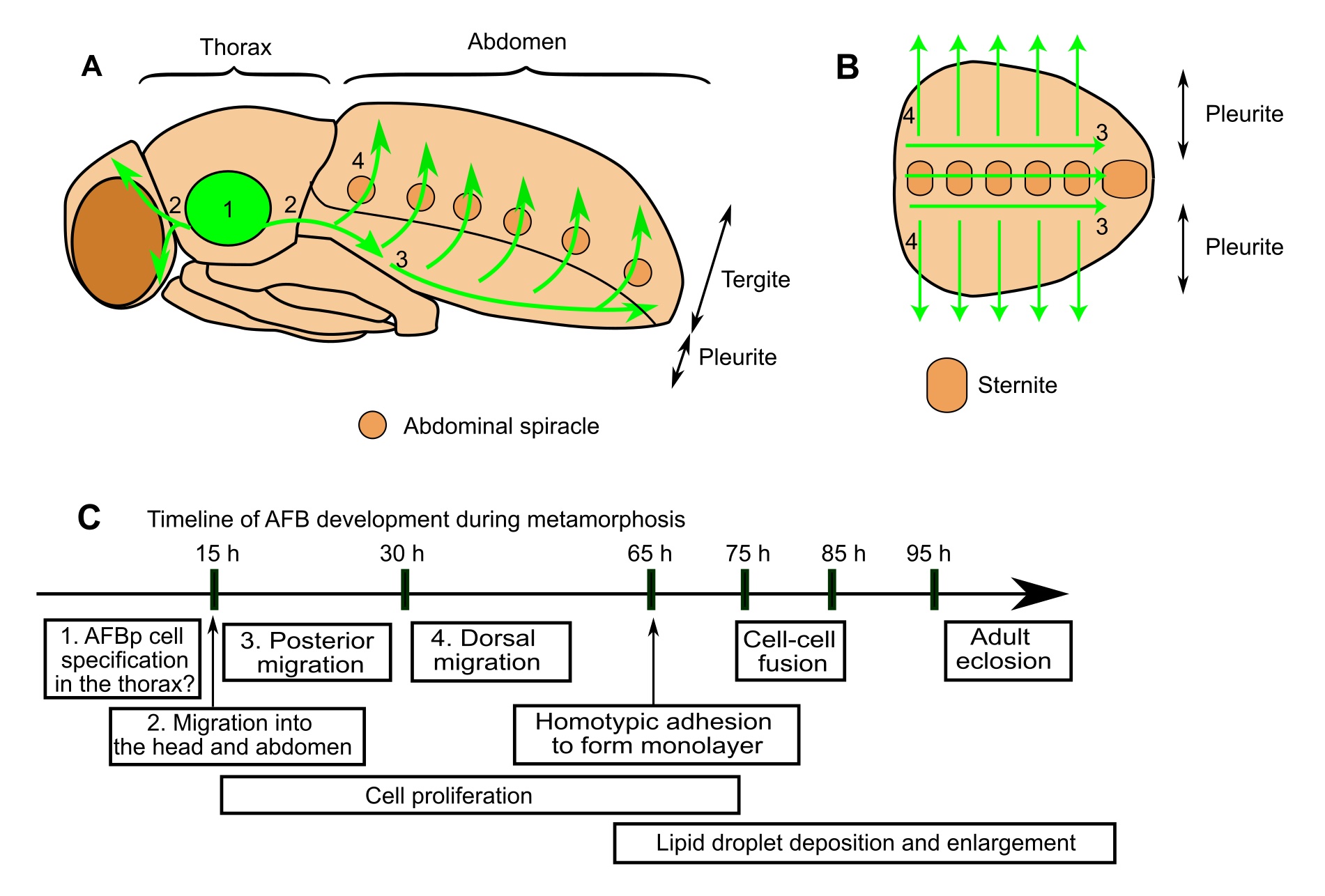
Schematic illustrations of AFBp migration pathways in the fly (A,B). While our data support the notion that AFBp cells originate from thoracic segments, the detailed site of origin is still unclear. (C) Timeline of fly AFB development during metamorphosis (h: hours after puparium formation). Numbers in text boxes refer to numbers in A and B.
Are you surprised that the adult fat body precursor cells have to migrate over a long-distance from the thorax to disperse across the body?
Yes. The migration-based distribution strategy makes a striking contrast with those of other mesodermal organs. Larval fat cells and muscle precursors differentiate in a segmentally-repeated manner in the embryo. Adult muscles, another class of mesodermal tissue that undergo metamorphic remodeling, are also locally generated during metamorphosis. Thus, such on-site differentiation is likely to be the canonical mechanism for the broad distribution of mesodermal organs in the fly; in contrast, the long journey of the AFB precursors appears to be exceptional.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
We experienced at least two eureka moments in our searches for Gal4 driver stocks that can induce gene expression in the AFB. After establishing new Gal80 lines, which block Gal4 activity in larval fat cells, we tested various known fat-body Gal4 drivers with our Gal80 lines and found that the c833-Gal4 driver could visualize migrating adult fat precursor cells. Then, in our additional search for Gal4 lines related to mesodermal genes, two svp Gal4 strains exhibited persistent Gal4 expression from early in the AFB lineage onward when used with lineage tracing tools. The moment when we saw the precursor cells migrating from the thorax into the abdomen was memorable indeed (Movie 2 in Tsuyama et al. 2023).
And what about the flipside: any moments of frustration or despair?
With the new Gal4-based tools, we started lots of imaging with fluorescent protein markers and got a rough picture of the developmental progression of the AFB. Then, we examined whether wildtype flies without protein markers start to deposit lipid droplets at the same stage using Nile Red (a lipid stain); however, we were puzzled to find that various wildtype flies exhibited reduction or absence of Nile Red-positive lipid droplets with variable degrees of penetrance late in metamorphosis. We suspected that fluorescent markers might affect the developmental timings of the AFB but finally found that various fly stocks, including a widely used wildtype strain Canton-S, showed disorganized or lost AFB tissues with approximately 30% (!) penetrance even in the mature adult stage. We first could not come up with such an idea that a major tissue is occasionally lost in wildtype flies. This finding prompted us to examine defects in AFB development in a collection of inbred lines, Drosophila Genetic Reference Panel, performed by Yusaku Hayashi. Our results strongly suggest that the aberrant AFB development observed in those wildtype genetic backgrounds might be due to genetic variations, and we are attempting to characterize novel candidates of genes controlling AFB development as our ongoing study.
Where will this story take the lab?
Tadashi Uemura: So far as a separate project, I have been studying long-term effects of nutritional environments during larval stages on the reproduction and lifespan of adult flies. The background of that project and a potential link with our study on AFB development are the following: massive and rapid growth of juveniles is heavily influenced by the quality and quantity of nutrients consumed. The impact of the nutritional environment in the early life (the nutrition history) is not restricted to that stage; the nutrition history exerts long-term effects on adult health in the later life. I have been investigating on underlying mechanisms including identification of the key cell where the history is stored, and the AFB precursor could be one of such candidate cells.
Taiichi, what brought to you join Prof. Uemura’s lab? And what is next for you after this paper?
When I was a senior undergraduate student, I studied molecular mechanisms underlying plant-microbe symbiotic interactions using Lotus japonicus (supervised by Dr. Shingo Hata; I never imagined that studying gut-microbe and plant-microbe interactions is so trending now!). Reading papers with whole plants brought up my interest on molecular genetics. I was also interested in morphogenesis of cells. At that time, Uemura-san’s lab had been studying the molecular basis of dendritic morphogenesis using fly molecular genetics. So, I thought it might be the best place to join as a graduate student. After some studies on how aberrant ATP metabolism due to mitochondrial dysfunction causes the loss of dendritic trees of sensory neurons as my doctoral project (Tsuyama et al., 2017), we started this study on the development of the AFB. Even though these projects took a whole lot of time, Uemura-san generously and continuously supported my thoughts and plans. Recently, I have been focusing on ATP metabolism by developing genetic tools to manipulate energetic metabolism and structural analyses of proteins related to ATP metabolism in Prof. Ken Yokoyama’s laboratory at Kyoto Sangyo University.
References
Lawrence, P. A. and Johnston, P. (1986). Observations on cell lineage of internal organs of Drosophila. Journal of embryology and experimental morphology 91, 251–66.
Hoshizaki, D. K., Lunz, R., Johnson, W. and Ghosh, M. (1995). Identification of fat-cell enhancer activity in Drosophila melanogaster using P-element enhancer traps. Genome 38, 497–506.
Shimono, K., Fujishima, K., Nomura, T., Ohashi, M., Usui, T., Kengaku, M., Toyoda, A. and Uemura, T. (2014). An evolutionarily conserved protein CHORD regulates scaling of dendritic arbors with body size. Scientific Reports 4, 4415.
Tsuyama, T., Tsubouchi, A., Usui, T., Imamura, H. and Uemura, T. (2017). Mitochondrial dysfunction induces dendritic loss via eIF2α phosphorylation. Journal of Cell Biology 216 (3), 815–834, jcb.201604065.
Tsuyama, T., Hayashi, Y., Komai, H., Shimono, K. and Uemura, T. (2023). Dynamic de novo adipose tissue development during metamorphosis in Drosophila melanogaster. Development 150 (10): dev200815.


 (No Ratings Yet)
(No Ratings Yet)