Wellcome PhD – Lab 3: Pigs that fly
Posted by Jonathan Lawson, on 8 December 2011
This is my personal report on the last of three laboratory projects which I have undertaken during the rotation year of my 4-year Wellcome Trust PhD. I studied how flies depend on Pigs to fly.
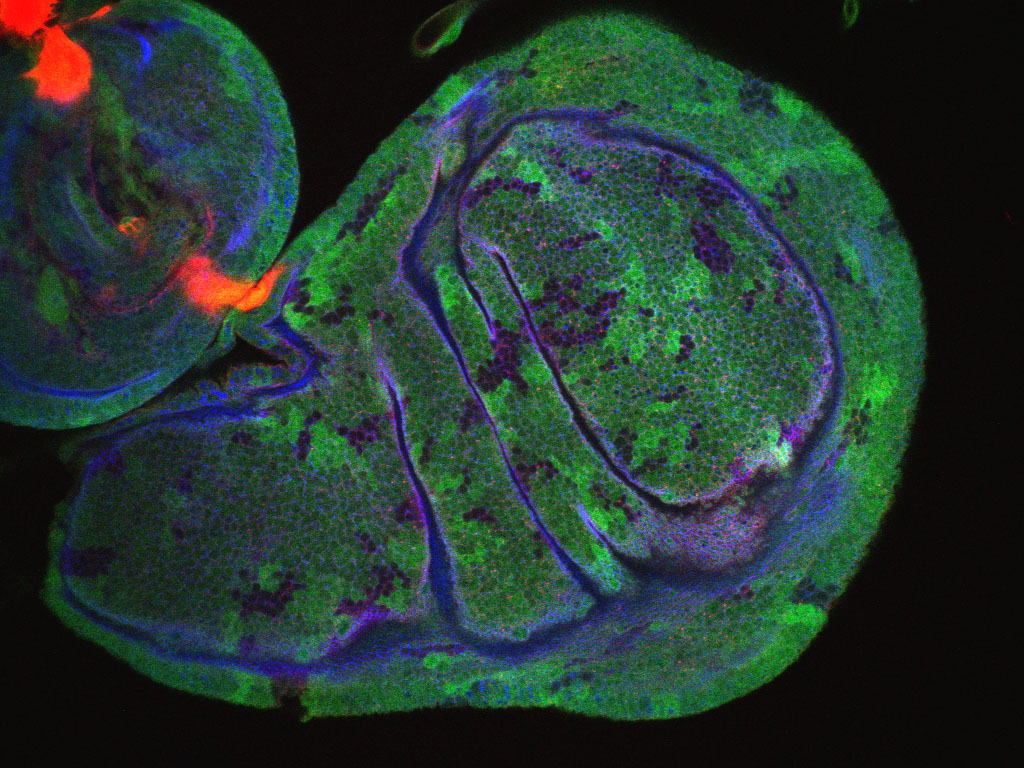
It is vital that the cells that make up your body’s tissues are correctly organised. If cells can’t differentiate between up and down (or inside and outside) they can’t possibly organise themselves into the complex structures we need to function. For the final rotation in the first year of my 4-year Wellcome Trust PhD I joined Dr Katja Röper’s lab to work with fruit flies (Drosophila melanogaster), investigating the complex and unusual nature of an organiser protein – the brilliantly titled ‘Pickled eggs’ (Pigs for short).
As I have discussed in some of my previous posts, cells contain a network of internal scaffolding known as the cytoskeleton, which helps to control cell shape and movement. It also provides transport routes to help move molecules and organise everything within a cell. Different parts of the cytoskeleton perform different functions, differentiated by the size of the structures they form and the proteins they are made of. The thinnest are microfilaments, made of actin, responsible for cell shape (they also allow muscles to work). The thickest are microtubules, made of tubulin, which form the major transport routes within a cell. Other animals (but not flies) contain further intermediate filaments that have primarily structural roles.
Although these different parts of the cytoskeleton have been studied independently for many years, it has only recently been discovered that they often work together. It turns out that there are many components needed to make these interactions possible. One of these components in fruit flies is the protein Pigs, a ‘GAS2-like protein’ related to four known human proteins. Pigs is thought to interact with both microfilaments and microtubules, forming a bridge that holds the different structures together.
What Pigs actually does is very poorly understood. Although the effects of its removal have been studied for several years, the results have been cryptic. Pigs is present in all of a fruit fly’s cells, but only at very low levels. However, in a few locations, including the developing wings, it is present at a much higher level. Loss of Pigs prevents the fly’s flight muscles forming correctly, preventing flight. In addition, female flies are sterile as the absence of Pigs prevents egg formation. However, it seems that widespread loss of this protein is needed to cause these changes, as the loss of Pigs in just a few cells does not produce the same effect. The function of Pigs is thought to be closely related to the Notch pathway, a cell-to-cell communication pathway, which may be responsible for the effects we have seen.
The Pigs protein consists of two halves – the first contains regions thought to be involved in binding to the cytoskeleton, with the second thought to help the protein associate with microtubules (although very little is known about it). I studied both the whole protein and its two halves separately. In flies I investigated the pigs gene, or its individual halves, to understand its role in wing development. I isolated either the gene, or its parts, and introduced them into bacteria, allowing me to produce large amounts of purified protein. This enabled me to study any interactions the protein may have with other purified proteins, particularly actin microfilaments and tubulin microtubules. In vitro experiments such as these have several limitations, as it is difficult to accurately reproduce the conditions found within living cells. This means that the proteins being studied may act differently under experimental conditions than they do in real life. However, in vitro work is a good way to investigate direct protein-protein interactions without other components of a living cell affecting the result.
Although I successfully introduced the pigs gene into some bacteria, I was unable to isolate purified Pigs protein within the timeframe of the project, so was unable to investigate its potential interactions. However, in parallel to this work I searched the existing literature for the sequences of GAS2-like proteins (including Pigs). For this work I developed a computerised method of analysing protein structures, which allowed me to perform some interesting in silico analysis of the proteins and gain useful indicators as to how we might expect Pigs to act.
Although similar tools are available online, this project provided a fun and engaging challenge while I wrestled with bacteria. The results led me to believe that fragments of the Pigs protein may be interesting, based on findings from related proteins. I decided to separate the microfilament binding region and the microtubule binding region of the protein to investigate what effects these parts of Pigs may have when separated that they do not have when combined. This added to the demands of the in vitro project but, having already overcome many problems with the original Pigs halves, was a much easier task.
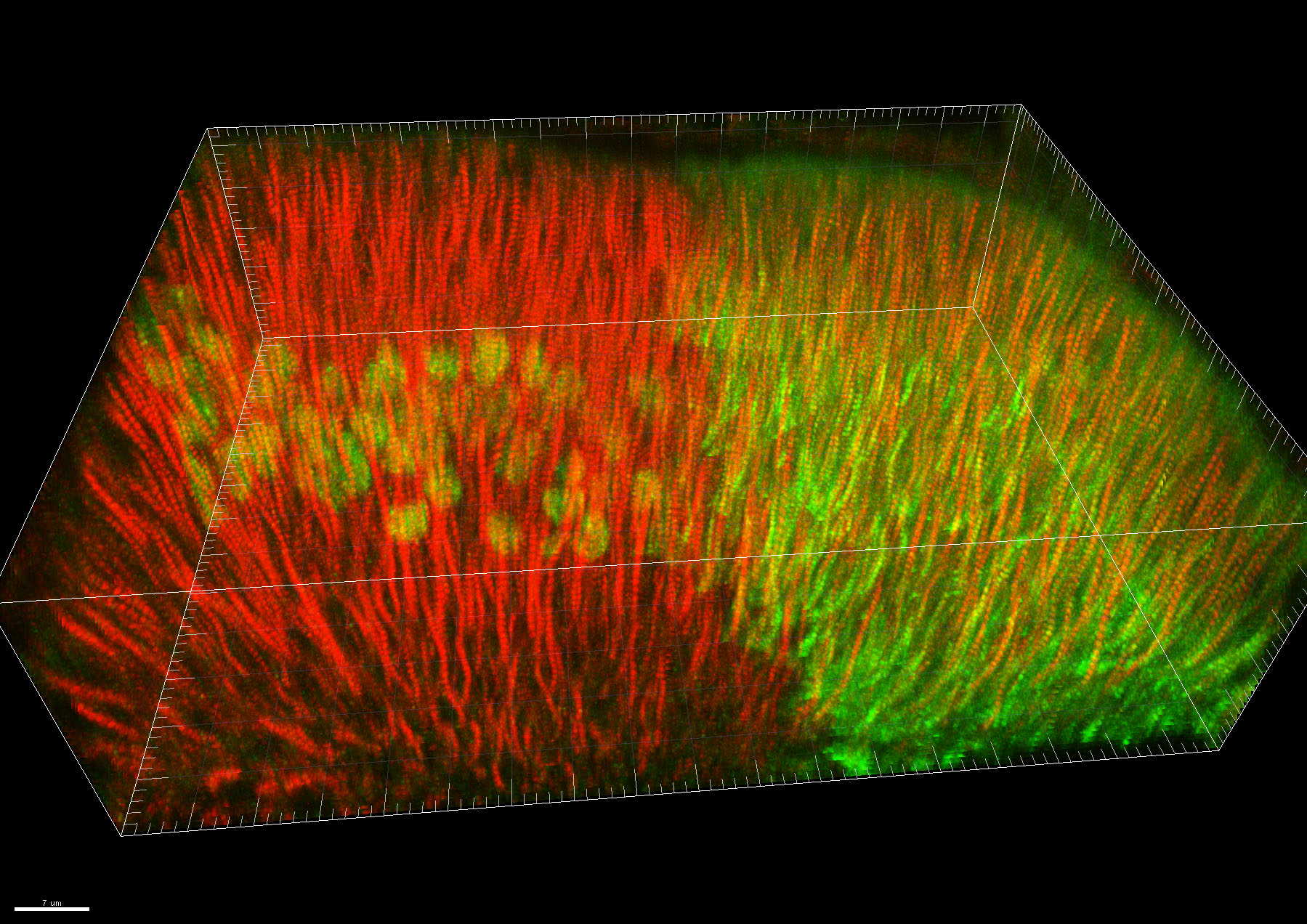
In another strand of work, we investigated wing development in fruitfly larvae just before pupation. We removed the pigs gene from some cells in the developing wing two days before studying it, creating groups of Pigs-less cells. Interestingly, there was no obvious difference between wing cells with and without Pigs, similar to what had been seen in experiments in other parts of the fly.
We also used a different system to increase the amount of full-length or partial Pigs in half of the developing wing. This is known as overexpressing a protein. Increasing Pigs levels had some interesting and unexpected effects on cell shape and internal organisation, although the explanation for how and why these changes occur is still unclear. We were unable to identify any changes when only half of Pigs was increased.
So that’s pretty much it. I also went to my first real conference during this project, the BSCB/BSDB spring meeting in Canterbury, which was a fantastic way to meet people from the field — especially many other inspiring young researchers. I have written about this in more detail here and here.
I have chosen to return to the Carazo Salas lab, from my second rotation for my PhD project. My thesis will be a genomic study of fission yeast, exploring the processes involved in organising cell polarity.
This post, and others about my PhD are also available on the Wellcome Trust blog here, here, here and here. I’ve also been doing some intermittent personal blogging here. For more about science in Cambridge checkout the science magazine, BlueSci.


 (3 votes)
(3 votes)