Gaining traction: What Hippos can teach us about vertebrate embryonic morphogenesis
Posted by David Kimelman, on 19 January 2018
In our recently published paper, we discovered that the Hippo pathway transcription factors have an unexpected role in creating the conditions for the zebrafish body to extend posteriorly during embryogenesis, as well to form the precursors of the dorsal and ventral fins. Here is the backstory of the twists and turns that lead to these results, and why we think it is important.
It began with an email…
One day almost two years ago, I got an email from Jason Lai, a grad student in the lab of my friend and colleague Didier Stainier, asking if I would be interested in studying a mutant they made using CRISPR. Didier’s lab does great work studying heart development, but this mutant also has an amazing effect on the formation of the embryonic body, which I study, and so they asked if I would be interested in pursuing it. The email contained a movie, which shows that the body starts to form normally, but then as the tail starts to grow out it suddenly collapses. I was so excited by this movie that I immediately wrote back to start the collaboration, without doing any background reading first.
Comparison of sibling (left) and yap1;wwtr1 double mutant (right) embryos. The movie begins at the 3-somite stage.
Jason and Didier’s mutant eliminates the function of two transcription factors in the Hippo pathway called Yap1 and Wwtr1 (a.k.a. Taz). Single mutants in either Yap1 or Wwtr1 have no effect on forming the body, but when both are mutated the posterior body fails to form normally as shown in the movie. When I started reading the literature, I sadly discovered that in the fish species medaka a very interesting Yap1 mutant called hirame had already been described a year earlier in a Nature paper. In this paper, the authors proposed a model in which an apparently ubiquitous Yap1, acting through the Rho GTPase Activating proteins (called Arhgaps), provides tissue tension that allows the embryo to oppose the forces of gravity since the hirame embryos look like they have partially collapsed along the dorsal-ventral axis1. The authors hadn’t examined the formation of the posterior end of the body, but in a figure showing a hirame embryo it clearly looked to me like the posterior body was defective, much as we were seeing in zebrafish. The evidence for the role of the Arhgaps was actually based on a human cell culture system and not on any strong evidence from studying hirame embryos. However, since my lab had been starting to investigate the role of Arhgaps in zebrafish, I thought we could at least add a tiny bit to the literature by figuring out exactly which Arhgap was involved downstream of Yap1 and Wwtr1 in the embryo.
Never discount the value of serendipity
As luck would have it, my department had just hired a terrific new Assistant Professor named Young Kwon, who is an expert in Yap1 in Drosophila. When I told Young about this mutant that I was starting to work on, he offered me an aliquot of a commercial Yap1 antibody he had. I didn’t think it would be very valuable since the hirame paper implied Yap1 was everywhere, but never being one to turn down a free reagent I gave it a try. The results really shocked me, since not only was the Yap1 localized, but it was localized to the skin and notochord (Fig. 1)! The reason this was so surprising to me was that the mesoderm is the engine that drives the elongation of the body as people like Ray Keller have shown for a long time, with the notochord only providing a minor component, whereas the skin is something that most of us just ignore. Jason then followed up by looking at Wwtr1 with an antibody, and found exactly the same localization. We also looked at medaka Yap1, and this was also in the same places, so this was not just some zebrafish weirdness.
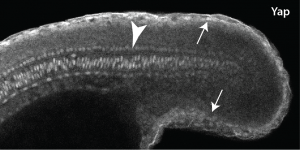
Not knowing what to do we turned to RNA-seq, since I hoped that levels of Yap1 and Wwtr1 that did not show up with the immunostaining would still be working to activate mesodermal genes. Jason did the RNA-seq experiment in Didier’s lab, and while the embryonic localization of most of our top hits (none of which were arhgaps) were not known, when I did the in situs I found that they were all expressed in the skin and notochord. Thus, there was no easy solution to this conundrum.
I get by with a little help from my friends
I was constantly puzzling about what the skin and notochord had to do with the formation of the body when I suddenly remembered some recent beautiful papers from another friend and colleague, Scott Holley2-4. Scott also has been interested in how the vertebrate embryo forms, and particularly the formation of the somites. Scott and his lab have shown not only that Fibronectin and Integrins are important in the morphogenesis of the embryonic body, but the very surprising result that while Fibronectin is secreted by all cells, it only assembles into extracellular matrix around the borders of each somite, much the way a pillowcase covers a pillow. Part of this Fibronectin matrix occurs where the somites touch each other (the intersomitic boundary), whereas the rest of the matrix is where the somites touch the skin and notochord. What really grabbed my attention was a line in one of the papers that the Fibronectin “matrix pattern gives rise to the tissue mechanics of the mesoderm required for body elongation”3. In other words, Fibronectin is providing an adhesive surface that allows the mesodermal engine to grab onto in order to be able to drive elongation of the body.
Scott kindly gave me the very detailed protocol for Fibronectin staining his lab had carefully worked out, and with this I could see that the Fibronectin matrix was present in yap1;wwtr1 double mutants, but clearly defective. Natalie, my lab manager, and I then examined normal embryos where we expressed a dominant-negative Fibronectin, and produced embryos that looked very much like the yap1;wwtr1 mutants. So if the issue was a failure of the mesoderm to attach to the skin and notochord we might expect to see adhesive defects between these tissues, and this is exactly what we saw. For example, imaging in live embryos we could see the skin rip away from the mesodermal tissue as the mutant phenotype got progressively worse, something we didn’t see in normal embryos (Fig. 2).
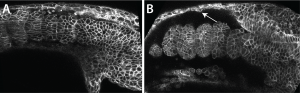
So what does it mean?
Our results show that the Hippo pathway transcription factors play a critical role in forming the posterior body of the early fish embryo by regulating a set of genes in the skin and notochord that allow the Fibronectin matrix to assemble normally at the border with the mesoderm. Thus, when the mesoderm starts elongating, it has something strong to grab onto and push against (imagine the difference between walking on a normal floor versus walking on one covered in oil). In addition, this Fibronectin matrix provides a surface for the skin cells themselves to undergo very interesting morphogenetic movements that Natalie elucidated, which allow the dorsal and ventral fins of the embryo to form (you will have to read the paper to learn more about that but it is summarized in Figure 3).
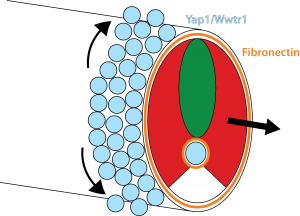
How all the Yap1 and Wwtr1 targets affect Fibronectin assembly is a big unanswered question. While the arhgaps are essentially unchanged in the yap1;wwtr1 mutants, of the many genes that do change, none are obviously known to affect Integrin and Fibronectin, so clearly there is much more to learn about this story.
References
1 Porazinski, S. et al. (2015) YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 521, 217-221.
2 Dray, N. et al. (2013) Cell-fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr Biol 23, 1335-1341.
3 Julich, D. et al. (2015) Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology. Dev Cell 34, 33-44.
4 McMillen, P. & Holley, S.A. (2015) The tissue mechanics of vertebrate body elongation and segmentation. Curr Opin Genet Dev 32, 106-111.


 (7 votes)
(7 votes)