The people behind the papers – Chloé Dominici & Alain Chédotal
Posted by the Node Interviews, on 18 January 2018
Vertebrate brain development is characterised by cell migration, as neurons are often born far from where they need to end up. Migration is regulated by guidance cues and their receptors, but, problematically, many of these molecules are expressed throughout the brain, complicating efforts to spatially and temporally pin down their function. A paper in the latest issue of Development addresses this problem using cell-specific knockouts for Slit and Robo, crucial migration regulators. We caught up with first author Chloé Dominici and her supervisor Alain Chédotal of the Institut de la Vision in Paris to find out more.

Alain, can you give us your scientific biography and the questions your lab is trying to answer?
AC Our lab studies the cellular and molecular mechanisms controlling the development of brain connections, mostly focusing on so-called commissural neurons which interconnect the right and left side of the nervous system. We mainly use genetics and mice but have also started to study other organisms (birds, amphibians, marsupials, humans etc) to address the question of the evolution of midline guidance processes.
I started to study the development of commissural circuits during my PhD in Paris, using various neuroanatomical methods including electron microscopy. For my post-doc, I wanted to learn other techniques such as genetics, molecular biology and biochemistry and joined the lab of Corey Goodman at UC Berkeley who had just discovered the first vertebrate Semaphorin. It was an exciting time as little was known about midline crossing in vertebrates. Within a few years all the main guidance cues, Slits, semaphorins, netrins, ephrins, and their receptors were characterized. After starting my own group, I continued to work on axon guidance, but also neuronal migration, angiogenesis and myelination. In parallel, we have been developing new 3D imaging techniques combining whole-mount immunostaining, tissue clearing and light-sheet microscopy, to facilitate the phenotypic analysis of our mutant mice.
And Chloé, how did you come to be involved with this project?
CD When I first joined Alain’s lab, my project was aiming to understand the role of Slit2 in the development of motorneurons. We initially attempted to do so by using conditional lines and wanted to show, as a control, that we could also remove Slits in the floor plate. After several attempts, I wasn’t able to remove Slits in the motorneurons but the conditional line for the floor plate worked very well. So, we began to analyse these mutants by focusing mainly on the hindbrain as it had been previously reported that Slits were involved in the migration of precerebellar neurons, a model system in the lab.
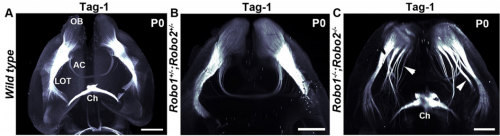
What were the limitations of previous genetic studies on the role of Slit and Robo in precerebellar neuron migration, and how did you set out to overcome these?
AC & CD The current model proposes that Slits act as repulsive factors for precerebellar neurons (PCNs), acting through Robo receptors. Accordingly, Slits are found at the floor plate, that most PCNs do not cross, and in the facial nucleus that one class of PCNs, the pontine neurons, avoid during migration. This conclusion was primarily supported by the phenotypic analysis of Slits and Robo knockouts in which many PCNs fail to stop at the midline or migrate over the facial nucleus. However, the existing lines were full knockouts. As Slits and Robos are expressed in many cell types and control the development of many organs, it was possible that some of the PCN migration defects were secondary to anomalies in other systems. To address this question, we have used conditional knockouts to selectively delete Slits and Robos from a limited number of cells. This was particularly challenging for Slits as there are three mouse orthologues with high redundancy. For instance, they are all co-expressed by floor plate.
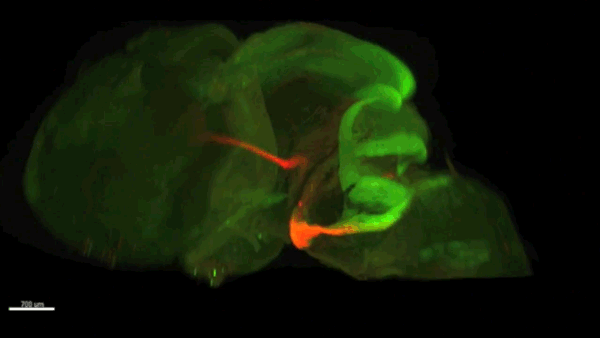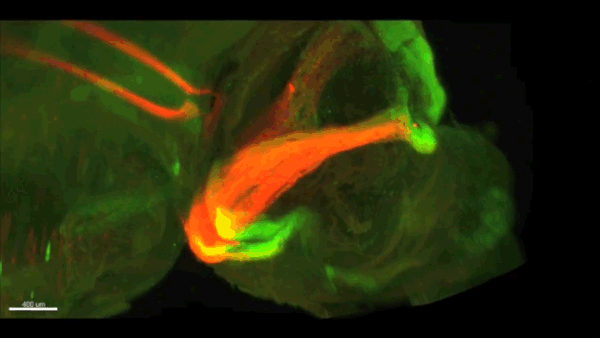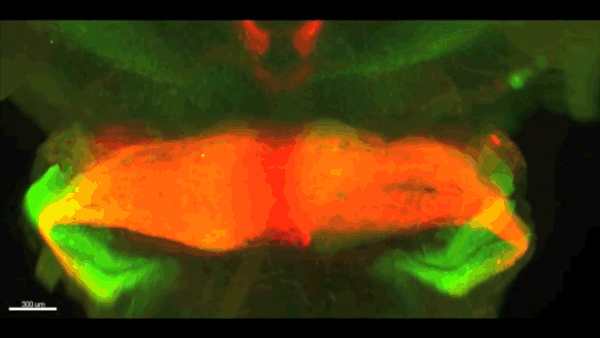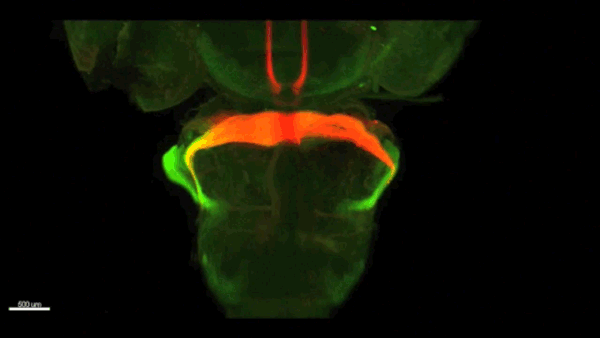
Can you give us the key results of the paper in a paragraph?
AC & CD We show that the floor plate-specific deletion of all Slits perturbs midline crossing of spinal cord commissural axons (many accumulate at the midline as expected from lower repulsion). But surprisingly, it does not have any noticeable consequence on the development of precerebellar neurons, which still stop at the midline. Likewise, the simultaneous deletion of Slits from the floor plate and the facial nucleus does not alter pontine neuron migration. Moreover, precerebellar neurons lacking Robo1and Robo2 receptors (the two main mediators of Slit repulsion) migrate normally. Together these results suggest that the migration of precerebellar neurons is independent of Slit/Robo signalling and that other guidance cues control midline crossing in this system. They also show that the mechanisms controlling midline crossing differ between spinal cord and hindbrain commissural neurons.
Do you have any clues as to which other signalling cues might be involved in stopping precerebellar neurons at the midline?
AC & DC Semaphorins are obvious candidates as they are repulsive and control midline crossing in the spinal cord as well as in the cortex. However, this is a large protein family (around 30 members), and in most cases conditional knockouts are not available. Semaphorins are also redundant and single knockout often display minor axon guidance defects. Moreover, none of the semaphorin knockouts that were analysed display PCN migration. Alternatively, Ephrins/Eph receptors are also good candidates and a previous study showed that some PCN neurons cross the midline in EphA 4mutants. However, I would rather support the existence of additional guidance cues, outside the usual suspects. In addition, it is likely that different guidance mechanisms control the migration of the different types of PCNs.
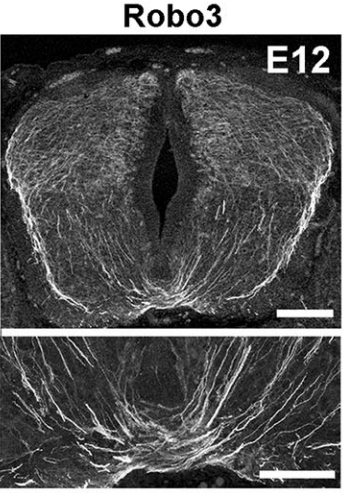
Last year you showed that Netrin-1 from the floor plate was not required for commissural axon guidance, contrary to long-held models, and now you’re proposing a re-evaluation of how Slit and Robo work. In both cases, did you deliberately set out to challenge established hypotheses, or did the results come about more by chance?
AC & CD Most midline guidance cues (Slits, Netrins, Semaphorins) are expressed in other cell types, including commissural neurons themselves. Therefore, even if the corresponding knockouts have clear axon crossing phenotypes, the direct demonstration of the floor plate as a main source of these factors was lacking. This pushed us to generate conditional knockout lines (Slit2 and Netrin 1) to selectively ablate them from specific cells. In addition, we wanted to use the mice to study the function of these molecules in the postnatal and adult nervous system, which is impossible with the classic knockouts which all die shortly after birth.
The increase in CNS size during vertebrate evolution has put commissural neurons at a larger distance from the floor plate, in particular in the hindbrain, which might explain why they might rely less on floor plate cues, at least initially. This might have been accompanied by a relocation of the main guidance cues to other cell types, closer to commissural neurons.
The imaging in your paper is stunning: can you give us an introduction to technique and how has it changed your view of developing nervous system?
AC & CD The 3DISCO-tissue clearing method was initially developed by Ali Ertürk and Frank Bradke who published it in 2011 in Nature Medicine. The basic principle is to use a series of solvents to solubilize lipids and homogenize the refractive index of the sample (such as a whole embryo, or an adult mouse brain). The method is fast, cheap and works with a wide array of tissues and species. Our addition to this field was to adapt whole-mount immunostaining on top of tissue clearing. Fluorescent dyes are not altered during clearing and samples can be quickly imaged in 3D using a light-sheet microscope. This has significantly facilitated and accelerated the phenotyping of our axon guidance mutants and also diminished the number of samples needed to understand the defects (as they can be oriented in all directions using a 3D Imaging software). We can also be much more precise in the evaluation of the phenotypes of compound mutants. Dissecting the embryo is not needed with this method, therefore you can also easily extend your analysis to organs that are outside the CNS, something that would be too time-consuming using classic histology.
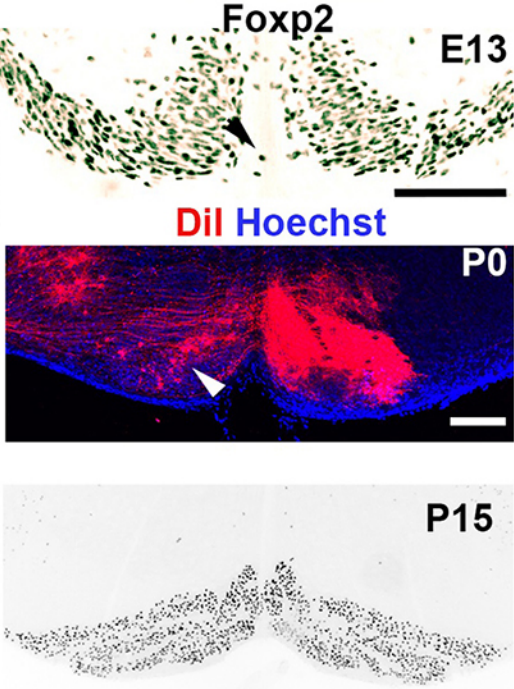
When doing the research, did you have any particular result or eureka moment that has stuck with you?
CD When I began to study motorneuron development, I wanted to visualize motor pools in 3 dimensions. This coincided with the emergence of the first tissue clearing techniques and commercial light sheet microscopes. We first used the 3DISCO clearing protocol, but GFP, the reporter used to label axons in our lines, quickly bleached after clearing. I wondered whether we could still visualise GFP using immuno-labelling. I decided to test an in toto immunostaining on a small portion of spinal cord and then cleared it, but I wasn’t really expecting to see anything. To my surprise, it worked! The images were incredible! We then optimised the technique and I could later apply the same technique to visualise migrating precerebellar neurons in 3D.
Not only were we able to visualise the migration of this neuronal population like never before, but we also were able to show that conditional Slits removal had no impact on precerebellar neuron migration.
And what about the flipside: any moments of frustration or despair?
CD Whilst it was a eureka moment, it was also a moment of despair when we realised that conditional removal of Slits in motor neurons showed no clear phenotype. Moreover, the generation of the mouse lines (more than 30) for my project was very difficult. In some mutants we had a one in sixteen chance of getting the right combination of genes. Indeed, the revision of this paper took almost a year due to the difficulty of obtaining additional mutants.
What next for you Chloé?
CD My PhD was quite intense. So I decided to take a year off after defending my PhD in 2016. I travelled and looked for multiple other opportunities. After taking a step back from research, I have realised that it is still something I am incredibly passionate about and would like to pursue. I am currently applying for postdocs.
Where will this work take the Chédotal lab?
AC Our work shows that the midline puzzle is not solved. Commissural neurons are highly diverse: some migrate to the midline, some cross it, others don’t, some project to both sides of the CNS and so on. How could a single unifying model account for this diversity? It has been considered for a long time that midline crossing mechanisms were similar in all bilaterians but this is not the case. We are now trying to identify new regulators of midline crossing using genetic screens, proteomics and transcriptomics. We are also comparing guidance mechanisms in multiple vertebrate species to determine if changes in receptors and ligands played a role in brain evolution, by modifying neuronal connectivity and migration. Some of our preliminary results seem to support this hypothesis.
Finally, let’s move outside the lab – what do you like to do in your spare time?
AC Cooking is one of my favorite hobbies and is the best method I know to rake my mind off science. This said, running an experiment and cooking a dish have a lot in common.
CD Unfortunately during my PhD, I had little time to do much extracurricular activities. But when I managed to squeeze some experiments, I took some time out of Paris to visit my family and friends. This really allowed me to take a step back from the stress of the PhD.
Chloé Dominici, Quentin Rappeneau, Pavol Zelina, Stéphane Fouquet, Alain Chédotal. Non-cell autonomous control of precerebellar neuron migration by Slit and Robo proteins.
This is #35 in our interview series. Browse the archive here.


 (3 votes)
(3 votes)