The people behind the papers – Marina Matsumiya & Ryoichiro Kageyama
Posted by the Node Interviews, on 19 February 2018
Vertebrate segmentation involves the periodic formation of somites from the presomitic mesoderm, in a manner controlled by oscillating gene expression (the oscillations of the segmentation clock must be one of the marvels of nature!). While in vivo work has provided a framework for studying the process, many aspects of segmentation dynamics are obscured in the embryo. A new Techniques & Resources paper in Development describes a stem cell-derived in vitro system for studying segmentation dynamics. We caught up with first author Marina Matsumiya and her supervisor Ryoichiro Kageyama of Kyoto University to hear more about the story.

Ryoichiro, can you give us your scientific biography and the questions your lab is trying to answer?
RK I became Assistant Professor of Kyoto University in 1989 and started a project about transcription factors that regulate mammalian neural development. We reported the basic helix-loop-helix (bHLH)-type repressor genes Hes1, Hes3, and Hes5 in 1992 and the bHLH-type proneural gene Atoh1 (Math-1) in 1995, and since then we have been characterizing the functions of these genes in neural development. We found that the antagonistic regulation between Hes and proneural genes controls neurogenesis and gliogenesis.
In December 1997, I was promoted to full professorship in the same university and continued the bHLH project. In the same month, I met Olivier Pourquié, when he visited my group in Kyoto, and we had discussion about his finding (just published in Cell) that the expression of chick hairy1, a Hes1 homologue, oscillates during somite segmentation. This discussion made me keep pondering on how such oscillatory expression with ultradian rhythms is possible and how such dynamic expression regulates the downstream events. In the 2000s, we reported that Hes1 expression oscillates in many cell types by negative feedback, and that this oscillation is important for cell proliferation. In parallel with this finding, we identified a new member of the Hes family, Hes7, and found that its expression oscillates by negative feedback in the presomitic mesoderm (PSM), thereby regulating the somite segmentation. These findings brought us to the field of the segmentation clock. Hes7 oscillations are synchronized under the control Notch signaling, and the oscillatory expression of the Notch ligand Delta-like1 is involved in the synchronized oscillations. However, the detailed mechanism of the synchronized oscillation with traveling waves in the PSM is still unknown, and we have been trying to answer this key question.
What is the status of developmental biology research in Japan?
RK Developmental biology research is well funded in Japan if a stem cell or regenerative research field is included. However, besides this field, it seems to be more difficult to get sufficient funding. I think that translational research-oriented funding is a general trend in Japan, as observed world-wide. Collaborations are very active and actually essential to many research projects of developmental biology. Grants supporting domestic and international collaborations are also available. In addition, Riken offers a service of generation of transgenic mice as a collaboration basis, and many researchers utilize this service. I do not know the exact number of labs in developmental biology in Japan, but the Japanese Society of Developmental Biologists (JSDB) has about 1200 members. This is a good number, compared to The Society of Developmental Biology (USA), which has nearly 2000 members.
However, I am a bit concerned about the recent trend. There used to be more than 300 poster presentations in the Annual Meeting of the JSDB, but last year, there were only 193, suggesting that the number of young researchers in the field is decreasing over the years. One reason is that there were only a handful of poster presentations of the stem cell or regenerative research field in the Annual Meeting of the JSDB, while many Japanese researchers participated in the ISSCR in Boston last year. I feel that (classic) developmental biology and (fancy) stem cell or regenerative research should go hand-in-hand, but in reality these two fields seem to go separately in Japan. It would be desirable to incorporate more stem cell researchers into the JSDB meetings to advance the field properly.
And Marina, how did you come to be involved with this project?
MM I was interested in the mechanism of somitogenesis, so I joined the Kageyama Lab as a master student. There were no PSM-like cell culture systems, and my colleague, Kumiko Yoshioka-Kobayashi, suggested me that such systems would be important to examine the detailed mechanism of somitogenesis. After discussion with Kumiko and RK, I decided to make a PSM-like cell culture system. At almost the same time, I found that Olivier’s group published a method to induce PSM-like cells from mouse embryonic fibroblasts (MEFs), so we first used MEFs produced from our Hes7 reporter mice. However, we were not able to induce Hes7-expressing cells, and we next tried mouse embryonic stem (ES) cells following the paper from the same group published in 2015, which focused on the induction of muscle fibers from ES cells. We also had a chance to learn an ES cell culture method from Mototsugu Eiraku at Riken CDB, who established a method to induce the eye cup from ES cells. By combining these methods, we succeeded in inducing PSM-like cells, which exhibited Hes7 oscillations.
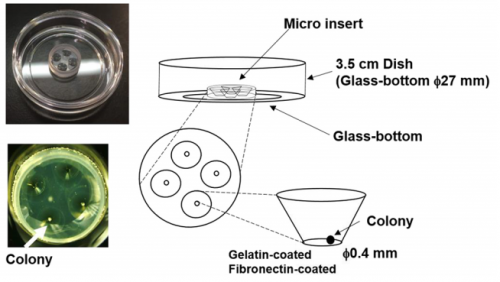
What are the difficulties of studying the segmentation clock in embryos? And what were the limitations of previous in vitro approaches?
RK Detailed analysis of the segmentation clock has been hampered because it requires the use of live embryos. Analysis involves both genetic and pharmacological approaches: in genetic approaches, mutant animals are generated to activate or inactivate gene functions, while in pharmacological approaches, either embryos or PSM tissues prepared from embryos are used for treatment with chemicals that modulate gene activities. If in vitro cell cultures are available, both genetic and pharmacological analyses would be facilitated, and many attempts have been made to induce PSM-like tissues from ES cells. Although PSM-like tissues have been successfully induced from ES cells, there are no reports of wave-like propagation of oscillatory gene expression in such induced tissues. So, we started the in vitro PSM cell project.
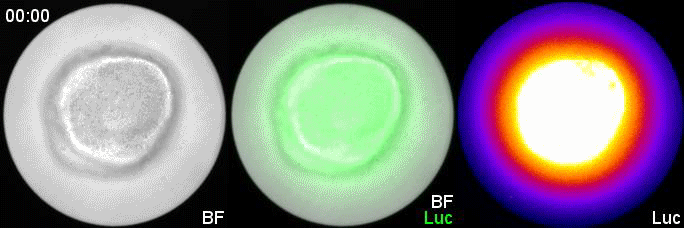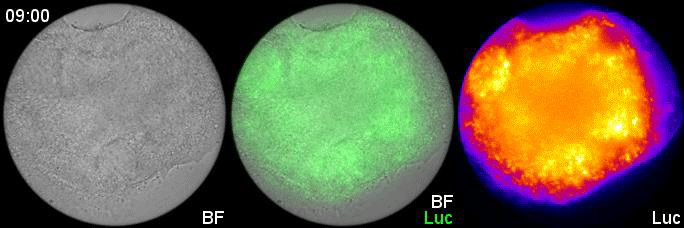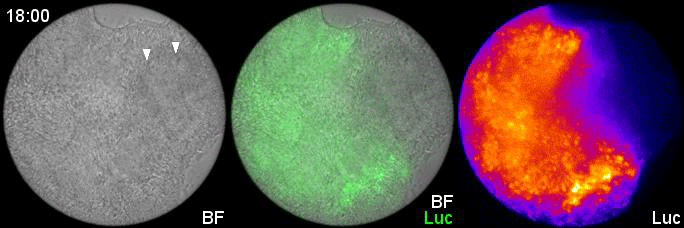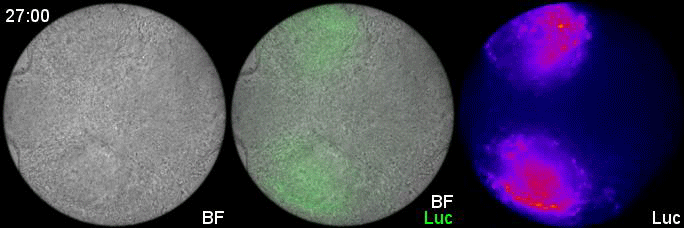
Can you give us the key results of the paper in a paragraph?
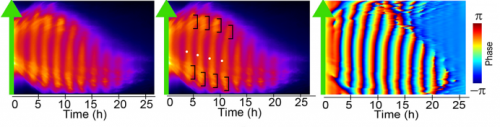
MM & RK We established a simple and efficient method to generate mouse ES cell-derived PSM-like tissues, in which Hes7 expression oscillates like traveling waves. In these tissues, Hes7 oscillation is synchronized between neighboring cells, and the posterior-anterior axis is self-organized as the central-peripheral axis, generating somite-like segments at the periphery. This method is applicable to not only chemical library and RNAi screening but also CRISPR-Cas9-mediated gene modifications and will facilitate the analysis of the molecular nature of the segmentation clock.
In your system, Hes7 oscillations did show some variability: do you know what underlies this variability?
MM & RK There is some variability in the amplitude, period, and patterns of Hes7 oscillations even in the same iPSM colonies, which may hamper to detect effects of chemicals and genetic modifications. This variability may depend on the size and shape of iPSM colonies, and how iPSM colonies expand on dishes. Further improvements will be required to reduce such variability.
Is your system restricted to mouse cells, or could you use it to compare segmentation between species?
MM & RK We have not examined other species yet, but we are sure that this method may be applicable to ES/iPS cells of other species. We are interested in the mechanism of how species-specific periods of Hes7 oscillations are determined.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
MM My eureka moment is when I observed Hes7 oscillation in iPSM colonies for the first time. I still remember the date, and we used the data in Figure 4 of the paper. We did not get nice data for a few years, but after changing the culture conditions, all of sudden, everything went well. We were surprised to see the Hes7 oscillations propagating like waves and leading to somite-like segment formation. When I saw the movie, we celebrated with high five. I could watch it forever. It took 6 months from the first detection of Hes7 oscillation to submit the paper. Every day in this half a year was a series of joy.
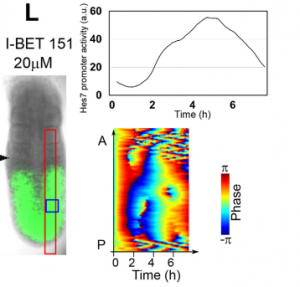
And what about the flipside: any moments of frustration or despair?
MM When I used MEFs, I was really nervous. I got some Hes7 signals but not oscillatory expression. I tried many different culture conditions, but none of them worked. It was like a never-ending nightmare, but now I feel that it is a very important step to improve myself mentally and technically.
What next for you Marina?
MM I have one more year to go before I finish my PhD course. Until then, I will try to reveal new factors or pathways that make Hes7 oscillations synchronized by using the iPSM method. This information will be important for the somitogenesis research. After finishing my PhD course, I would like to do research on refractory diseases of bone formation related to somitogenesis. Now, I will prepare the application of a fellowship to become a postdoc.
Where will this work take the Kageyama lab?
RK By using this system, we now started screening many chemicals to find a new pathway involved in synchronization of Hes7 oscillations. We also plan to use two-color fluorescent reporters (one from Hes7 promoter and the other from another important PSM gene) to examine the oscillation at the single-cell resolution. We are trying to find chemicals that do not repress Hes7 expression but affect synchronization. These chemicals, if found, would help reveal the mechanism of synchronized oscillation besides the Notch pathway. We have recently developed an optogenetic gene induction system and plan to introduce this system to iPSM cells. This system allows precise spatiotemporal control of gene expression, so we would like to see how light-controlled gene expression affects Hes7 oscillations.
Finally, let’s move outside the lab – what do you like to do in your spare time?
MM I would like to sleep, but I also like playing modern ballet. These are very important for me to control my condition.
RK I like jogging along the Kamo river in Kyoto, which really refreshes me. I also like walking. I used to bike, but after an accident I stopped biking now.
Marina Matsumiya, Takehito Tomita, Kumiko Yoshioka-Kobayashi, Akihiro Isomura, Ryoichiro Kageyama. ES cell-derived presomitic mesoderm-like tissues for analysis of synchronized oscillations in the segmentation clock. Development 2018 145: dev156836
This is #36 in our interview series. Browse the archive here.


 (2 votes)
(2 votes)