Synthetic Materials for Human Organoid Generation and Wound Healing (The Journey)
Posted by Ricardo Cruz-Acuña, on 20 November 2017
Looking back on the journey of: Ricardo Cruz-Acuña and Miguel Quirós et al. Nature Cell Biology (2017)
The Start
On August 2013, I took my first one-way trip departing from Puerto Rico. Although I have always been passionate about travelling to as many places (the cheapest way) possible, embracing the PhD-journey in a new city I would have to call “Home” was very intimidating. Close to the start of my journey at the Georgia Institute of Technology I joined the Garcia Lab due to my interest in the engineering of materials to study tissue development and regeneration. A couple more months later and I have already found myself very engaged in a new project in collaboration with the Nusrat Lab. The project involved the engineering of a synthetic hydrogel to direct mouse intestinal stem cell differentiation in vitro and serve as an in vivo cell-delivery vehicle that promotes intestinal wound repair, overcoming the translational limitations associated with natural matrices. This project aimed to provide potential therapeutic options by facilitating intestinal mucosal wound repair which is central in many pathologic states, such as inflammatory bowel disease.
At the time I was very much enjoying the work and, considering my heavy fundamental engineering background, it was all very novel to me as I faced a steep learning curve of very exciting biological concepts. In addition, having such amazing collaboration with cell biologists and clinicians placed me in the forefront of multiple fields that collide for a single goal.
It was all fun and games until I found myself two years later stuck in the same first-stage of my project because the mouse intestinal tissue did not survive within (or basically, hated) my hydrogels. As I continued to assimilate that we weren’t going to reach our desired experimental results (or in colloquial words, that “the project wasn’t working”) we met Jason Spence. He introduced us to the novel technology of in vitro generation of human intestinal organoids (HIOs) from human pluripotent stem cells (hPSCs). We grew to what is now a three-lab interdisciplinary collaboration involving bioengineers, clinicians, cell biologists and developmental biologists. And that’s where this journey took a different turn.
With experiments happening mostly at the University of Michigan I now had the opportunity to merge into another new environment with new collaborators and, just as significant, a different weather. Must say that for a Caribbean islander (despite having such amazing hosts) spending winter in Ann Arbor made the experience quite more challenging. Furthermore, due to the complexity of the hPSC culture I used to travel to Michigan in very short (sometimes even with one-week) notice. Thus, having to figure out the logistics for the trip (from lodging to shipping reagents; from all I was leaving behind in Atlanta to the experimental planning in Michigan) was very challenging. Once I was there, there was no time to waste. The clock was ticking, Miguel and I had an experimental plan to accomplish despite any (or the many) complications we faced and, to me, returning to Atlanta saying, “I didn’t have enough time to do that” was not an option. Nevertheless, after several trips to the beautiful (and cold) Ann Arbor, lots of planning and experimenting, research started to move forward, my project kept evolving and, most importantly, “it was working”…

The Science
In this project we studied the in vitro generation of human intestinal organoids (HIOs) from human pluripotent stem cells (hPSCs). This technology offers strategies for generating multi-cellular 3D structures that recapitulate important features of the human intestine. For example, HIOs can be used for the establishment of chronic intestinal disease models, such as inflammatory bowel disease (IBD), and provides a platform for functional modeling and repair of genetic defects in intestinal development. As part of the method to generate HIOs, hPSCs must be cultured and differentiated in a MatrigelTM-coated substrate, giving rise to 3D intestinal spheroids which are collected and encapsulated within MatrigelTM for expansion into HIOs. MatrigelTM suffers from lot-to-lot variability and an undefined tumor-derived nature which limits its clinical translational potential. Therefore, we implemented a synthetic hydrogel based on a four-armed, maleimide-terminated poly(ethylene glycol) macromer (PEG-4MAL), developed in the Garcia Lab, as a substitute to MatrigelTM for encapsulation of intestinal spheroids and further generation of HIOs. Our synthetic material offers significant advantages over natural materials due to its well-defined structure, cytocompatibility and minimal toxicity in vivo, overcoming the limitations of MatrigelTM.
In order to generate the HIOs using our synthetic hydrogel, floating intestinal spheroids were collected from the culture plate and encapsulated within PEG-4MAL macromers that were functionalized with adhesive and crosslinking peptide motifs for expansion into HIOs. The controlled stoichiometric incorporation of peptides to the synthetic material allowed me to independently modify the biophysical and biochemical properties of the hydrogel to ultimately identify an engineered material that supports in vitro generation of HIOs from hPSC-derived spheroids without the need of MatrigelTM. Both biophysical and biochemical properties of the synthetic matrix were important to intestinal organoid formation, and allowed me to engineer an optimal formulation that supports intestinal spheroid survival, expansion and epithelial differentiation into HIOs and differentiation into mature intestinal tissue in vivo to similar levels as MatrigelTM (Figure 2).
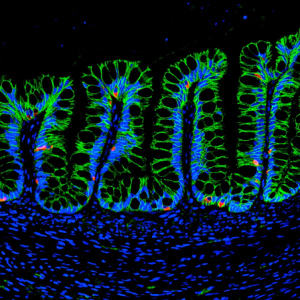
In addition (and probably my favorite part of the project), we established the use of the engineered hydrogel as a delivery vehicle for HIOs to murine intestinal mucosal wounds using a murine colonoscope. The tunable reaction time scales of this material allowed injection of hydrogel liquid precursors and HIOs to mucosal wounds resulting in an in situ polymerized hydrogel that supported localized organoid engraftment and enhanced wound repair. These findings form a basis for the development of HIO-based therapies to treat gastrointestinal diseases in humans involving intestinal epithelial wounds (e.g., IBD).
The (More) Questions
As we have established a synthetic hydrogel to generate hPSC-derived HIOs and treat intestinal injuries, many new questions arise: Can we use this material to study matrix contributions to gastrointestinal disease and repair? Can we use this material as an HIO-delivery vehicle to treat chronic gastrointestinal diseases? Can we establish this material as a platform to generate different types of human organoids? And the journey continues…
The Thanks
Now back in (the warmer weather of) Atlanta, I could go on for about three paragraphs naming everybody and everything that somehow contributed to what is, so far, my major achievement research-wise. Nevertheless, I feel is more than fair to primarily thank my homeland, Puerto Rico, and the University of Puerto Rico at Mayagüez, as they are the foundation of where I am today and to where I am going. I will forever be in debt to my island (that little piece of heaven) and will always hold it in my heart, proudly, wherever I am.


 (2 votes)
(2 votes)