Three dimensional human lung tissue in a dish
Posted by Briana Dye, on 6 May 2015
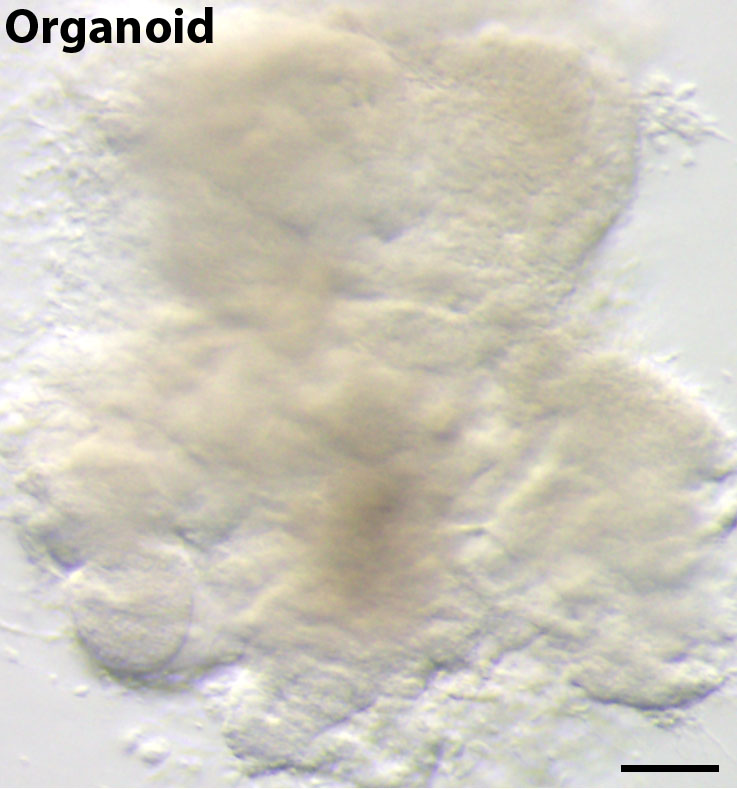
Pioneering efforts by others have made enormous strides in our ability to generate human lung tissue from human pluripotent stem cells (hPSCs); however, these efforts have largely focused on deriving lung-specific cells as flat monolayer cultures or growing these cells on scaffolds 1-7. In our paper, published recently in the open access journal eLife 8, we defined conditions that mimic key stages of lung development in vitro in order to direct differentiation of hPSCs into lung tissue. Importantly, without using engineering approaches such as scaffolds, these conditions prompted the formation of 3D structures in the tissue culture dish. These 3D structures, called spheroids, self-organized from the 2D monolayers and detached from the surface of the tissue culture dish. Spheroids started as small aggregates of epithelium and mesenchyme that were expanded into larger tissues, called human lung organoids (HLOs) (Figure 1).
Figure 1. A human lung organoid, generated from human pluripotent stem cells. Scale bar represents 100µm.
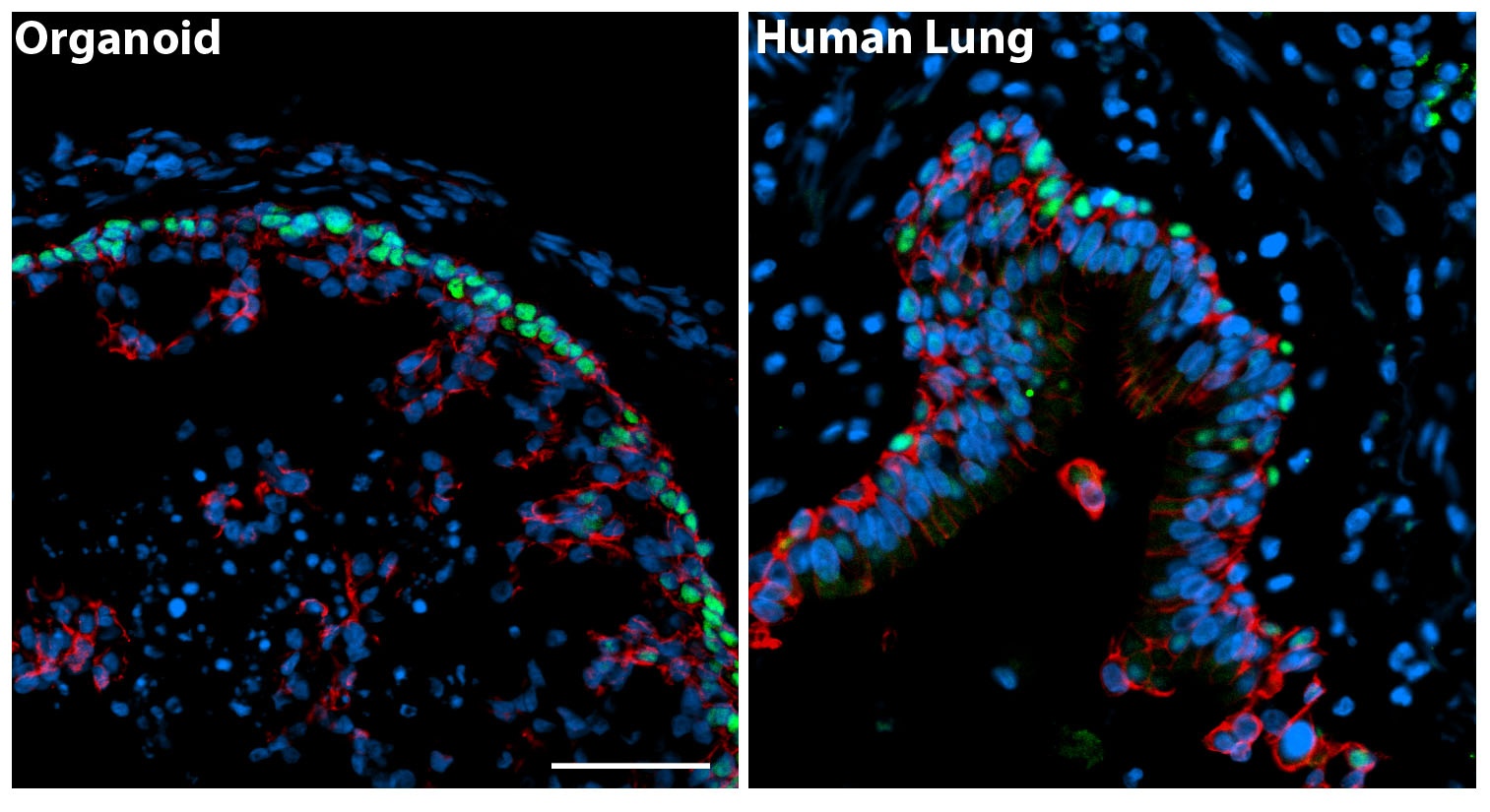
HLOs have structures that are similar to those found in a human lung (Figure 2). The human lung epithelium makes up the proximal airways, including bronchi and bronchioles and the distal alveoli where gas exchange occurs. Surrounding these epithelial structures is the lung mesenchyme, which includes smooth muscle and other cell types of support cells. Consistent with the human lung, HLOs had proximal airway-like structures that resembled bronchi. The HLOs also possessed distal progenitors and some mature alveolar cells (Type I and Type II), but have not yet formed the saccular structure of alveoli. The HLOs also possessed mesenchymal cell types, included smooth muscle cells and fibroblasts that surrounded both the proximal airway-like structures. This system, which represents the complex structural organization of the lung, coupled with diverse cell types, will allow us to begin to ask questions about human lung development, homeostasis, and disease pathogenesis.
Figure 2. The panel on the left is a cross section through an organoid and on the right a cross section of a human airway way. By immunofluorescence we detected basal cells (green, P63 marker) in the organoids that were organized in a similar manner to the adult airway. The epithelium is labeled in red (beta-Catenin) and all the nuclei in blue (DAPI). Scale bar represents 50µm.
Starting with human pluripotent stem cells, we added ActivinA, which mimics Nodal signaling, a required pathway for differentiation of endoderm, to derive definite endoderm over the course of 4 days. Previous studies have shown that the definitive endoderm is capable of differentiation into foregut endoderm when TGFβ and BMP signaling are inhibited by the small molecule SB431542 (SB) and Noggin, respectively 9. The foregut gives rise to a variety of organs including lung, thyroid, liver, and pancreas. We found that adding SB and Noggin for four days caused the cultures to express lung and thyroid markers. Although the cells acquire the appropriate fate markers, they failed to self-organize and remained as a monolayer. Drawing from previous research, we added a Wnt agonist and FGF4 to cue the formation of 3D clusters in the dish. Activating Wnt and FGF signaling causes the cells to undergo “morphogenesis” in the culture dish, resulting in self-organizing cell clusters called spheroids that detach from the monolayer and float in the media 10,11. Treating endoderm with a combination of TGFβ and BMP inhibitors plus WNT/FGF stimulation led to the formation of self-organizing 3D spheroids that had the appropriate foregut endoderm fate.
Lastly, in order to generate lung specific tissue, we induced Hedgehog (HH) signaling using a Smoothen agonist, SAG. All together, definitive endoderm was given the following instructive cues: (1) TGFβ and BMP inhibitors to derive foregut endoderm, (2) factors inducing 3D spheroid tissue formation (WNT and FGF4), and (3) SAG, a small molecule stimulating HH signaling which enhanced lung lineage induction. These factors were added daily over the course of 6 days and the self-organizing spheroids that floated in the media were collected starting on the 4th day of treatment. These foregut spheroids possessed both epithelium and mesenchyme and expressed foregut and lung-specific markers including SOX2 and NKX2.1 respectively. The floating spheroids were collected and placed in a 3D extracellular matrix, Matrigel. Next, foregut spheroids were expanded into lung organoids through the addition of FGF10 to the media that overlaid the Matrigel droplet. FGF10 is a critical growth factor during lung development and adult homeostasis and we found that it is essential to maintain a healthy epithelium in long-term cultures. Every two weeks the lung organoids were placed in a fresh droplet of Matrigel.
After 65 days in culture (D65), the HLOs had proximal airway-like structures containing cells expressing markers of specific cell types found in this region of the lung including basal, ciliated and club cells. These proximal airway-like structures were surrounded by mesenchyme containing fibroblasts, myofibroblasts, and smooth muscle. In addition to these proximal-like structures, the D65 organoids expressed distal cell markers of both alveoli progenitors and mature alveoli cell types that had similar morphology to the adult alveoli cells, Type I and Type II alveolar cells.
Collectively, we observed that HLOs cultured over 65 days possessed some mature cellular features; however, some cellular features appeared to underdeveloped, leading us to hypothesize that HLOs were more similar to fetal tissue than adult. To address this, we used global transcriptional profiles obtained using RNA-sequencing, and performed an unbiased comparison of HLOs to fetal and adult lung. We found that the derived lung organoids closely resemble fetal lung. It is possible that HLOs remain fetal since they are grown in dish and lack several components of the human lung including blood vessels, nerves, and immune cells. Ongoing studies are aimed at understanding additional cues and cellular inputs to mature the tissue.
In summary, HLOs possess both developmental progenitors and differentiated cells along with structures that resemble the native human lung. Human lung organoids will be an important tool to study human lung development, adult homeostasis, and disease pathogenesis and we are excited for the many new avenues of research this system opens up.
Kadzik, R., & Morrisey, E. (2012). Directing Lung Endoderm Differentiation in Pluripotent Stem Cells Cell Stem Cell, 10 (4), 355-361 DOI: 10.1016/j.stem.2012.03.013
Longmire, T., Ikonomou, L., Hawkins, F., Christodoulou, C., Cao, Y., Jean, J., Kwok, L., Mou, H., Rajagopal, J., Shen, S., Dowton, A., Serra, M., Weiss, D., Green, M., Snoeck, H., Ramirez, M., & Kotton, D. (2012). Efficient Derivation of Purified Lung and Thyroid Progenitors from Embryonic Stem Cells Cell Stem Cell, 10 (4), 398-411 DOI: 10.1016/j.stem.2012.01.019
Mou, H., Zhao, R., Sherwood, R., Ahfeldt, T., Lapey, A., Wain, J., Sicilian, L., Izvolsky, K., Lau, F., Musunuru, K., Cowan, C., & Rajagopal, J. (2012). Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific Cystic Fibrosis iPSCs Cell Stem Cell, 10 (4), 385-397 DOI: 10.1016/j.stem.2012.01.018
Wong, A., Bear, C., Chin, S., Pasceri, P., Thompson, T., Huan, L., Ratjen, F., Ellis, J., & Rossant, J. (2012). Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein Nature Biotechnology, 30 (9), 876-882 DOI: 10.1038/nbt.2328
Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, & Niklason LE (2013). Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. The Journal of clinical investigation, 123 (11), 4950-4962 PMID: 24135142
Huang, S., Islam, M., O’Neill, J., Hu, Z., Yang, Y., Chen, Y., Mumau, M., Green, M., Vunjak-Novakovic, G., Bhattacharya, J., & Snoeck, H. (2013). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells Nature Biotechnology, 32 (1), 84-91 DOI: 10.1038/nbt.2754
Firth, A., Dargitz, C., Qualls, S., Menon, T., Wright, R., Singer, O., Gage, F., Khanna, A., & Verma, I. (2014). Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells Proceedings of the National Academy of Sciences, 111 (17) DOI: 10.1073/pnas.1403470111
<Dye, B., Hill, D., Ferguson, M., Tsai, Y., Nagy, M., Dyal, R., Wells, J., Mayhew, C., Nattiv, R., Klein, O., White, E., Deutsch, G., & Spence, J. (2015). In vitro generation of human pluripotent stem cell derived lung organoids eLife, 4 DOI: 10.7554/eLife.05098
Green, M., Chen, A., Nostro, M., d’Souza, S., Schaniel, C., Lemischka, I., Gouon-Evans, V., Keller, G., & Snoeck, H. (2011). Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells Nature Biotechnology, 29 (3), 267-272 DOI: 10.1038/nbt.1788
Spence, J., Mayhew, C., Rankin, S., Kuhar, M., Vallance, J., Tolle, K., Hoskins, E., Kalinichenko, V., Wells, S., Zorn, A., Shroyer, N., & Wells, J. (2010). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro Nature, 470 (7332), 105-109 DOI: 10.1038/nature09691
McCracken, K., Catá, E., Crawford, C., Sinagoga, K., Schumacher, M., Rockich, B., Tsai, Y., Mayhew, C., Spence, J., Zavros, Y., & Wells, J. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids Nature, 516 (7531), 400-404 DOI: 10.1038/nature13863


 (3 votes)
(3 votes)
Beautiful study! I am fascinated by the system’s ability to generate both lung epithelium and lung mesenchyme, lineages that in vivo originate from different germ layers!
I’m the third author on this paper. In light of the above comment, I might as well add that the endoderm we generate is not 100% pure, which likely explains the origin of the mesenchyme.