The people behind the papers: Adam Davis, Nirav Amin and Nanette Nascone-Yoder
Posted by the Node Interviews, on 13 April 2017
In spite of our external appearance, our innards are asymmetric. For today’s interview, we feature a paper published recently in Development that provides a cellular and molecular investigation into symmetry breaking in a poorly understood organ, the stomach. We caught up with first authors Adam Davis and Nirav Amin, and their supervisor Nanette Nascone-Yoder, Associate Professor in North Carolina State University, Raleigh, NC.

Nanette, can you give us your scientific biography and the questions your lab is interested in?
NN-Y I have been interested in left-right asymmetry for a long time. I did my graduate work with Mark Mercola at Harvard Medical School, studying the induction and left-right asymmetric patterning of the heart. At NC State University, we’ve been investigating the morphogenesis and evolution of the gastrointestinal tract, specifically, how the embryonic gut tube forms the requisite three-dimensional shape necessary for proper physiological function, including appropriate length and left-right asymmetric curvature.
And Nirav and Adam, how did you come to join Nanette’s lab?
NA During my career, my research interests have focused on the transcriptional control of cell fate specification and organ development. I did my PhD work in the lab of Dr. Jun “Kelly” Liu at Cornell University, where I focused on the transcription factors, and their targets, that controlled muscle and non-muscle fates in the C. elegans mesoderm. From there, I wanted to apply the tools and techniques I used in C. elegans to the classic vertebrate model for developmental biology, Xenopus. I did my postdoctoral work in the lab of Dr. Frank Conlon at the University of North Carolina at Chapel Hill, where my research focused on a transcription factor, Casz1, and its role in cardiac development. As part of my project, I also participated in some RNA-seq and ChIP-seq studies to identify targets of Casz1 in the heart. Eventually, I began exploring methods to determine the functional roles of genes in cardiac development by editing frog genomes via mutagenesis and transgenesis.
Towards the completion of my postdoctoral work, I met Nanette and she told me about a research project in her lab in which she was using a non-model organism, the Budgett’s frog, to investigate how organs establish left-right asymmetry. These frogs have large embryos (three times larger than the Xenopus embryo!), and she was using this to her advantage in some transcriptomic studies. My experience with this type of data and functional studies in the frog made me a perfect fit for the project. I was excited by the possibility of developing this frog as a new model for developmental biology research and I joined the lab three years ago.
AD I’ve always been interested in animals, particularly in how they are shaped. I’ve also always been interested in how our DNA shapes our organs and organ systems. I performed my PhD work in the lab of Dr. Ed Stellwag at East Carolina University. I studied how Hox genes are regulated in the developing brain and pharyngeal arches. I used the Japanese medaka (Oryzias latipes) as a model to study the development and evolution of morphology. I enjoyed learning how transcription factors and their respective cis-regulatory elements regulate gene expression during development. I first met Nanette when I presented a poster of my research at the Southeast regional conference meeting for the Society of Developmental Biology at UNC, Chapel Hill in 2007. I was amazed by her talk and the research her lab was performing at NC State, particularly in how genes influence cellular morphogenesis. Once I finished my Ph.D., I was lucky enough to obtain a postdoc position in her lab under a NIEHS training grant. I was excited to work with Pitx2, as it is a homeodomain-encoding transcription factor. I was also excited to understand how organs are shaped at the cellular level.
You reference some papers from the 1960s hypothesising as to how the stomach gets its curvature. Why do you think it has taken until 2017 for the first experimental investigation into its cellular and molecular basis?
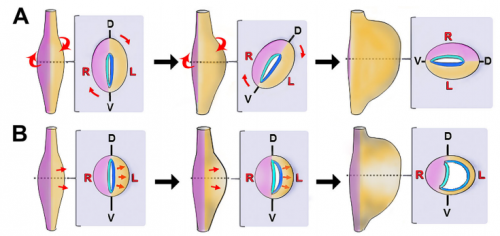
NN-Y The rotation theory was actually put forth in the late 19th century, based on retrospective observations of human embryos. Rotation of the organ nicely explained the final anatomical location of left and right nerves along the front and back of the stomach; hence, the idea of early organ rotation became widely accepted and propagated such that it became dogma in all the textbooks. We were certainly not the first to question this model. Dissenting theories have been proposed by other scientists before now, but these studies were also based largely on retrospective studies in human embryos, and conducted prior to modern molecular developmental biology.
It is important to clarify that the stomach probably does undergo rotation events during later development, in order to refine the final contour and position of the organ as it descends into the abdominal cavity. However, our results indicate that wholesale rotation is not the mechanism of establishing the initial LR asymmetric curvature, as has been assumed for over a century.
What led you to address this question using both mice and frogs?
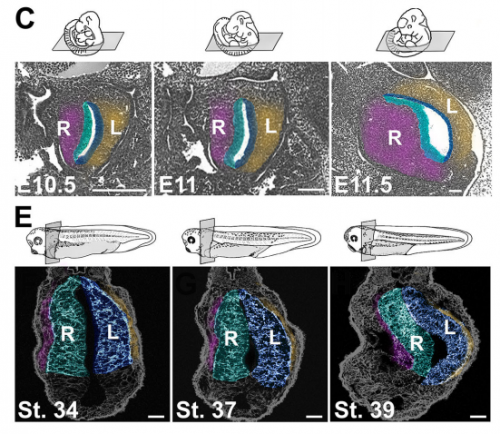
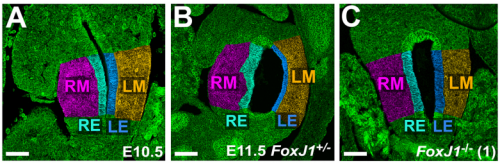
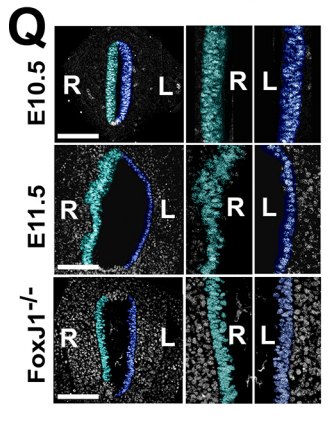
NA One day Nanette pulled me into her office to show me some pictures publicly available on emouseatlas.org. It was clear that a lot of the observations she and Adam had made in Xenopus held true in the mouse stomach. We were fortunate that the Ghashghaei lab here at North Carolina State University had the Foxj1 mouse mutant, enabling us to directly test how curvature is affected when left-right asymmetry is randomized, and to show that the origins of stomach curvature are conserved in mammalian development.
Can you give us the key results of the paper in a paragraph?
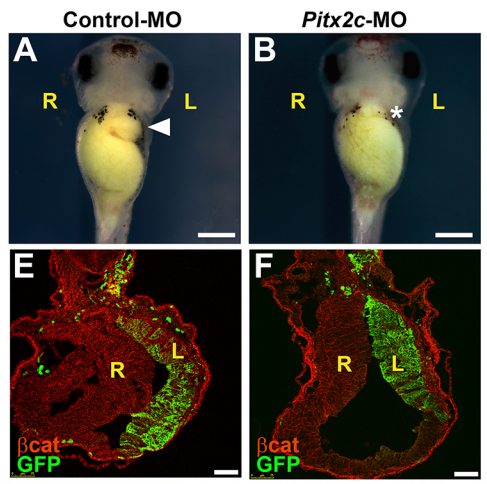
NA The J-shaped stomach is characterized by a longer “greater” curvature and a smaller “lesser” curvature. Historically, this shape has been thought (and taught) to be the result of a rotation of the dorsal surface of the gut tube to the left. However, others have suggested that this curvature is not the result of rotation, but an intrinsic asymmetric growth of the stomach to give rise to the greater curvature. In this paper, we use frog and mouse embryos to show that the latter hypothesis is true –differential lengthening of the left wall drives the curvature of the stomach. This lengthening occurs due to radial rearrangement which cause the left stomach wall to thin. Importantly, this process is dependent on Foxj1/Nodal/Pitx2c, key determinants of left-right asymmetries in multiple organisms.
Do you have any idea how Pitx2 is directing stomach morphogenesis?
NA Our working hypothesis for how Pitx2 is directing stomach morphogenesis is through the regulation of cellular effectors that drive radial intercalation. Our current research focus is in the identification of such factors within the left stomach.
Are all asymmetric internal organs made asymmetric in different ways? How does the stomach relate to the rest of the digestive system, for instance?
NN-Y Different types of morphological asymmetries form in different organs. For example, some organs form an acute curvature like the stomach or early heart tube. Other organs adopt left right asymmetric positions, or undergo asymmetric regression or remodeling, such as the spleen and vasculature. In others, grossly different morphologies develop out of their left versus right halves/ counterparts; examples include the cardiac atrial chambers or the contralateral lobes of the liver and lungs. Unfortunately, for the majority of organs, we know surprisingly little about the cellular and molecular events that break symmetry during organogenesis, so it is unclear whether these varied types of morphological asymmetries may form in different ways.
The recent work in intestinal rotation does provide one point of comparison. The appearance of cellular differences between the left and right sides of the dorsal mesentery breaks the symmetry of the structure that suspends the gut tube from the body wall, leading to a leftward shift in gut position, ultimately biasing the direction of intestinal rotation. Interestingly, no developmental asymmetries are thought to exist in the intestine itself. In contrast, we find that the left and right sides of the foregut tube itself undergo distinct morphogenetic processes to drive stomach curvature. At the cellular level, left side cells are more polarized /organized than right side in both stomach and mesentery; however, at the tissue level, the left stomach wall thins and expands, while left mesentery condenses. Only the mesoderm layer of the gut is involved in the mesentery, while both mesoderm and endoderm become asymmetrical in the stomach. So there are both similarities and differences in the development of asymmetry in each context. In the future, we will need to look at a variety of organs in order to determine the degree of universality or divergence in organ-level symmetry breaking.
When doing the research, was there a particularly exciting result or eureka moment that has stayed with you?
NA For me, I was excited by the success of the CRISPR reagents I generated to look at Pitx2c function in the stomach. Though it wasn’t the most crucial data pertaining to this paper, it was ground-breaking for me in that I could use CRISPR/Cas9 to systematically interrogate gene function in the F0 generation for Pitx2 and other genes I identify to be involved in asymmetry. This has proven to be a very time- and money-saving finding.
AD Absolutely! It was when I was examining immunochemically-stained transverse sections of wild-type Xenopus stomachs at several developmental stages. I noticed that several morphometric factors (E-cadherin, aPKC, and γ-tubulin) showed much more robust apical localization in the left endodermal cells of the developing stomach than the right. This was observed in developmental stages prior to gut curvature at the gross anatomical level.
And what about the flipside: any moments of frustration or despair?
NA Having worked with worms and frogs, I have gotten used to being able to follow embryogenesis in real time and in large numbers of animals. When we embarked on the mouse side of this project, the frustration for me came in not being able to 1) get more than 2-3 mutant animals per litter and 2) precisely control the timing of when we get embryos. On the bright side, it made me appreciate the advantages of Xenopus even more!
AD There were a lot more of these than the eureka moments, including antibodies not working properly, needles clogging when trying to inject embryos, frogs not yielding viable eggs, me wanting to pull my hair out, etc., etc.
What next for you following this work?
NA I am very excited to continue this work – we have used transcriptome profiling in the Budgett’s frog to identify some interesting genes that function together with Pitx2 in regulating stomach curvature. Hopefully soon, you’ll be reading more about these!
AD Thanks to my experience with my PhD, and as a postdoc in Nanette’s lab, I am currently a tenure-track assistant professor at Gordon State College. I enjoy teaching Developmental Biology and Human Anatomy and Physiology. I learned a wealth of information regarding molecular and developmental genetics, cellular and gross morphogenesis, anatomy, histology, and microscopy. I used this information to build my developmental biology course. I also enjoy training undergraduate students in research in developmental biology.
And finally – what do you get up to when you are not in the lab?
NA The majority of my non-lab life is dedicated to my wife, 4 year-old daughter, and 2 year-old son. They keep me balanced and make me feel young (and old at the same time!). Now that the weather is getting nicer here in North Carolina, we are constantly outside staying active – we have started our garden and will be camping, biking, and more in the coming months.
AD My non-academic life is dedicated to my wife, Rebecca, and our 7 year-old son, Finn. Like Nirav, they give me balance with my work life. We’re near the Appalachian Mountains, so we love hiking and camping. Finn and I enjoy searching for and photographing salamanders and other wildlife when we hike. Also, last summer, we made our first insect collection together.
Back to you Nanette – where will this paper take your lab?
NN-Y We are currently identifying the cellular morphogenetic events that underlie symmetry-breaking in two other organs. As Nirav mentioned, we have also devised a novel strategy for identifying the molecules involved, using a novel model organism (the Budgett’s frog). The hope is that these genes could be candidates for human organ defects. Variation in these processes may also be involved in generating novel organ anatomy and morphology during evolution.
, , , , ,
Browse the People Behind the Papers archive here


 (3 votes)
(3 votes)