Actin and microtubules link to form a tube within a single cell
Posted by Sofia Araújo, on 14 December 2020
In the Genetics of Cell Behaviour in Development laboratory, at the Department of Genetics, Microbiology and Statistics at the University of Barcelona, we work with the terminal cells (TCs) of the tracheal system of Drosophila melanogaster, which are very interesting cells. During embryonic development, they are able to generate a tube within their cytoplasm, a so-called seamless, subcellular tube. As the larvae hatch and grow these tiny tubes are able to branch inside a single cell to provide oxygen to the various tissues in the animal.
Embryonic and larval tracheal TCs are a powerful model to understand the mechanisms that lead a single-cell to change its shape, and generate a subcellular seamless tube, in a process analogous to capillary blood-vessel sprouting. Cell elongation and subcellular tube formation are dependent on dynamic cytoskeletal reorganization. Microtubules and actin are major players in this active cytoskeletal process. We have been studying the initial events in the formation of this subcellular tube, focusing on what triggers these changes in the cytoskeleton.
When we investigated the role of centrosomes as Microtubule Organizing Centres (MTOCs) in TCs (Ricolo et al., 2016), we became interested in analysing microtubule bundles inside TCs. We tested the tracheal overexpression of several constructs consisting of MT-associated proteins fused with fluorophores. Among them, we used transgenic lines overexpressing the Drosophila spectraplakin Short-stop (Shot).
Shot is a giant multifunctional protein responsible for many aspects of cytoskeleton organization in different tissues. Shot can operate as a single cytoskeleton component, can coordinate cytoskeletal elements such as actin and microtubules, and can also interact with other cellular structures. The functional versatility of Shot is probably reflected by the abundant generation of different isoforms, and by the characteristics of its protein domains. The full-length isoform A (Shot-A) contains an NH2-terminal actin-binding domain (ABD), different central domains (rod-like domain, plakin spectrin-like repeats and an EF-hand), and a C-terminal domain involved in MT interaction (GRD and C-tail domains). Shot-A has been shown to act as an actin-microtubule crosslinker. Isoform C (Shot-C) lacks a proper actin-binding domain and has been described to interact mainly with MTs (Voelzmann et al., 2017).
Since we were interested in visualizing MTs in TCs in live embryos, we tested the tracheal overexpression of Shot-C using the transgenic line UAS-shotC-GFP. This was a clear winner! GFP staining of embryos overexpressing Shot-C-GFP, allowed us to beautifully label MTs; we could clearly see the MT-fibres inside the TCs and they were bright enough that we could image them live, as the embryo develops.
But… (there is always a but). When you want to see a biological structure through the use of an overexpression system in Drosophila embryos, as in other organisms or cell culture systems, you have to be very careful. Often the overexpression of a specific protein can perturb the physiological state of the cell; in other words, it can give rise to a mutant phenotype. So, we decided to analyze if the embryonic overexpression of Shot caused any changes during TC extension and lumen development.
Surprisingly, we found that Shot overexpression induced the generation of an extra-subcellular lumen inside the TC, a very similar phenotype to the one caused by extra centrosome numbers. So, by searching for ways of illuminating MTs, we had serendipitously found another way to induce luminal branching at embryonic stages. Of course, our next question was whether Shot was inducing higher numbers of centrosomes in TCs. Nicely, we were able to genetically prove that Shot acted in a different way to generate the extra-subcellular lumen branching event.
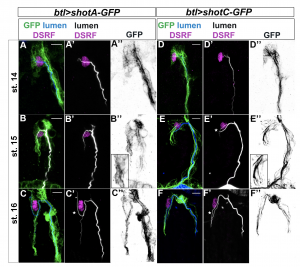
The results of our experiments told us that the more Shot we expressed in the TC, the more subcellular branching we could generate. So, how does this Shot overexpression generate branched subcellular lumina? One hypothesis was that an excess of MT-stabilization could induce the generation of acentrosomal branching points along pre-existent microtubule bundles along the luminal track. If this was true, the overexpression of other proteins involved in MT stabilization should be also able to induce extra subcellullar lumina. We tested this hypothesis and found that overexpression of the microtubule-associated protein Tau was able to generate the same luminal phenotype.
Analyzing the complete loss-of-function of shot we observed defects in luminal formation and cell elongation. We observed, using rescue experiments and analysis of endogenous Shot localization that this phenotype depended on Shot’s ability to crosslink MTs and actin. Surprisingly, we were able to rescue the shot null phenotype overexpressing Tau indicating a role for Tau in mediating the connection between actin and microtubules in TCs.
With our work, we showed the importance of actin-microtubule crosslinking in the formation of novel subcellular tubes by single-cells (Ricolo and Araújo, 2020). But also, this work has taught us that by doing research you can open new exploratory paths in an unpredictable way. Serendipity plays a role in science. You just need to keep an open mind and follow your curiosity!
Delia Ricolo and Sofia J. Araújo


 (7 votes)
(7 votes)