The people behind the papers – Daniel Osborn, Kuoyu Li and Simon Hughes
Posted by the Node Interviews, on 10 November 2020
This interview, the 81st in our series, was published in Development earlier this year.
A crucial step in vertebrate muscle development is the activation of myogenic regulatory factors (MRFs) that direct myogenesis. A new paper in Development investigates the roles of Fgf signalling and Tbx transcription factors in zebrafish MRF induction. We caught up with the paper’s two first authors, Daniel Osborn and Kuoyu Li, and their supervisor Simon Hughes, MRC Scientist and Professor of Developmental Cell Biology at King’s College London, to hear more about the story.

Simon, can you give us your scientific biography and the questions your lab is trying to answer?
SH After studying biochemistry as an undergrad and doing a PhD on rhodopsins with Martin Brand in Cambridge, I did a postdoc with Martin Raff in UCL on cell lineage in the optic nerve. In 1987 it was difficult to study cell lineage in vivo in the central nervous system, and the molecules that controlled cell fate were completely mysterious. But MyoD was discovered that year, so I switched to skeletal muscle, doing a second postdoc with Helen Blau at Stanford on how myoblasts generate slow and fast muscle fibres in specific patterns in each muscle. All three advisors taught me so much about both science and life. Before I moved back to London, I worked for a few months in David Botstein’s large group. We were sequencing the yeast genome, a precursor to the Human Genome Project and one of the first high-throughput ‘big science’ projects in biology; really exciting, but not my kind of science. In 1992, I joined the Medical Research Council Biophysics Unit at King’s College London, where Franklin and Wilkins had done their DNA structure work. It was an ideal place for me – Nigel Holder and Roger Patient had just set up a Developmental Biology Research Centre, and my lab linked that to the MRC Unit. We initially worked mainly on mouse and chick muscle, but Nigel soon inveigled me into zebrafish and I fell in love with the simplicity and ability to watch tissue development in real time. I was really lucky to meet, learn from, and collaborate with Monte Westerfield and Phil Ingham and their colleagues, whose differing approaches helped me combine genetics with our developmental cell biology. We have remained true to our original question of understanding the molecular genetics of muscle tissue patterning, though much of our work now focuses on later developmental stages, when zebrafish muscle is growing from committed muscle stem cells. I am really pleased with Dan and Kuoyu’s paper because, although it had a 20 year gestation, I think it fills in a missing link between patterning of early mesoderm and muscle.
Daniel, how did you come to work in Simon’s lab?
DO I started in Simon’s lab as a research technician back in 2001 where my primary role was running the then MRC/KCL zebrafish facility as well as contributing to the group’s research. It was a fantastically encouraging environment for a young biology graduate and I was immediately immersed in exciting developmental biology, the bright lights of London and my first salaried position. I loved the lab work and to fuel my interest Simon offered me a part-time PhD and KCL agreed to waive my bench fees. This sent me down the academic research career path and I haven’t looked back since. My thesis looked at the regulation of myogenic bHLH proteins during zebrafish slow muscle development. A substantial amount of work came out of it, contributing to four papers, and it is now with great pleasure that this collaborative effort has produced a fifth, and perhaps final, paper stemming from my thesis, which was completed 12 years ago.
How are each of you coping in the current COVID-19 pandemic?
DO I am now based at St George’s University of London where I run my own zebrafish group (my group is still very much interested in muscle development, but I am more of a gene hunter these days). Everything is now in stasis until the pandemic passes. It has been difficult to be away from the lab – we have a number of manuscripts in their final stages that need experiments completing before submission and it is frustrating not being able to get these done. Although experiments have stopped, I am still supporting undergraduate students and courses that are now running online, and it’s the time of year for marking dissertations. I also have three young children thrown into my daily mix. They are kindly humouring me by allowing me to explain to them genetic variation using Lego (different coloured bricks for different traits), perform crude DNA extractions from strawberries, and help them mount samples from around the house/garden on slides for viewing down our microscope. Luckily the weather has been surprisingly good, so we have been out in the garden exercising, home-schooling and indulging in plenty of fresh air.
KL I now work in a laboratory in the China Zebrafish Resource Center in Wuhan, having moved back at the end of 2010. By chance, I left Wuhan 2 h before the city was shut, to unite with my family for Spring Festival. At that moment, we didn’t realise how serious this pandemic would be. In our small town in Hubei we self-quarantined at home for 49 days. We ordered supplies online and volunteers brought them to our door every 2-3 days. The institute was shut, with no staff in the labs. Part-time staff came briefly every 4 days to feed the fish. Now, I am back in Wuhan and preparing to return to work next week (30 March). We hope the city will be back to normal after 2 months cold shock.
SH Experiments have been completely shut down and one of my team is recovering from COVID-19, but our many lines of fish are still happy, as far as I am aware. Just like Kuoyu’s, our Biological Services staff are doing a heroic job in very difficult circumstances. My family has escaped to rural Wales, so for me it’s email, FaceTime and Microsoft Teams between spring birdsong and isolated walks – quite idyllic. I’ve volunteered to run PCRs in local hospitals, but no call yet. I feel for my colleagues stuck in small flats in London as we await the approaching medical storm.
Let’s get back to the paper then – what led you to study the roles of Fgf and Tbx in myogenesis?
SH I had known about the importance of Fgf in mesoderm patterning since the work of Kimelman and Kirschner in 1987, and then the finding by my KCL colleagues Kevin Griffin and Nigel Holder that Fgf signalling was important in zebrafish trunk myogenesis. Kevin and Nigel proposed there was a gene they called ‘no trunk’ (by analogy with ‘no tail’, which is what the zebrafish T gene was called at the time). Kevin went as a postdoc to Dave Kimelman’s lab and showed that no trunk really existed; it was tbx16. Tbx16 is mainly known for controlling gastrulation movements at trunk levels; it was originally discovered as a mutant called spadetail that lacks most dorsal trunk tissue, and Sharon Amacher and Chuck Kimmel had shown this nicely. During the course of our study, both Dave Kimelman’s and Sharon Amacher’s labs showed that various Tbx genes collaborate in the formation of dorsal mesoderm. And Stephen Devoto’s lab had also shown that Tbx6, a close relative of Tbx16, is a negative regulator of presomitic mesoderm myogenesis. So it was really a no-brainer to examine this in our myogenic context, particularly with Fiona Wardle as a neighbour.
DO When the work began nearly 20 years ago almost all the MRF loss-of-function analyses had been done in mouse. So it was unclear how general the role of MRFs was in vertebrate myogenesis, particularly bearing in mind that Myod is not required for most myogenesis in flies. Our early experiments used morpholinos because that was the cutting-edge technology at the time; nowadays, we would do it by CRISPR genome editing. I was excited to see that zebrafish MRFs were initiated in the absence of Hh signalling in anterior somites, just as our lab had seen working with Betsy Pownall in Xenopus and as Andy McMahon had shown in smoothened mutants in mouse. Strikingly, just as Anne-Gaëlle Borycki showed in mouse, I saw that Hh signalling was more important in more caudal somites, so I asked what other signalling might be important to turn on the MRFs in anterior somites. SU5402 had been found to block Fgf signalling and it worked. Monte Westerfield’s lab found and published the same thing before us, which was both disappointing and encouraging, and meant we had to do more to publish something meaningful. So, I had shown with loss- and gain-of-function experiments that Fgfs in the tailbud and in the base of the notochord were important for myogenesis. It was one chapter in my thesis – I left at this point and Kuoyu took over the project.
KL When I joined Simon’s lab, we wanted to pursue two aspects of Dan’s thesis. I looked downstream of MRFs at how they drove slow myogenesis; the first paper Dan and I published together was on how Cdkn1c (p57) cooperates with Myod to drive slow myogenesis. But I also began looking at how Fgf might activate MRF transcription. I found that MRFs were no longer induced by Fgf signalling in tbx16 mutants and in morpholino conditions, and that Fgf signalling cooperates with Tbx16 to drive MRF expression, which again argued that both Fgf and Tbx16 are needed within the presomitic tissue to initiate MRF expression and myogenesis.
SH At that point, the project came to a halt. But luckily, Fiona Wardle and colleagues had data on Tbx16 and Tbxta binding to myf5 and myod that moved the project forward.
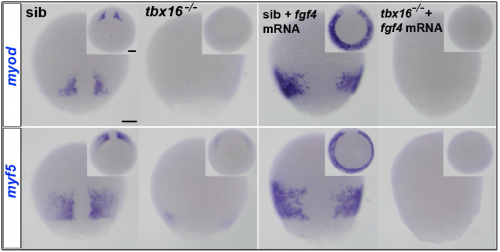
Can you give us the key results of the paper in a paragraph?
The key result is that, in addition, to their role in paraxial mesoderm migration, Fgf signalling from the tailbud midline triggers Tbx16 to bind and directly activate the myf5 and myod genes. The same goes for Tbxta on the myod gene, and that initiates slow muscle fibre formation adjacent to the base of the forming notochord. Combined with Andrew and Fiona’s data on Tbx binding to the MRF loci, this encouraged Steve Cutty to use the glucocorticoid receptor-Tbx16 fusion protein plus dexamethasone to show that binding triggers myf5 and myod transcription directly, without new protein synthesis from other genes.
What do you think explains the differences in transcriptional targets between Tbx16 and Tbxta?
DO, KL & SH That’s an interesting question. The consensus binding sequences don’t seem to differ, so presumably their differential binding at certain sites in myf5 and myod has to do with collaborating accessory proteins and/or local chromatin structure. But another issue is whether they are competing for binding at sites where we detected both bound; in the embryo tbx16 and tbxta are expressed in only partially overlapping cell populations, so they could do different things in separate cell types. There is also the possibility that cell signalling and post-translational modifications could make them differentially active, even when bound to the same site. There’s a lot to work out if one wants to fully understand it.
What do your findings suggest about the evolution of vertebrate musculature?
DO, KL & SH It’s now clear that Tbxt/6/16 family genes are required in all major chordate lineages for myogenesis in the body and tail (but not the head). We think this is likely the ancestral way muscle was made in vertebrates. But several kinds of muscle are made in the somites of all vertebrates, and we think diversification of this Tbxt/6/16 gene family allowed the evolutionary diversification that gave vertebrates their advantage. For example, Tbxt genes are famous now for specifying notochord and thus controlling midline Hh expression. We think it is no coincidence that Hh induces both muscle and motoneuron diversification and that in the most primitive extant chordate, Amphioxus, notochord has muscle character. Perhaps the evolutionary origin of notochord was a special kind of dorsalmost muscle.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
DO For me, the eureka moment that catalysed this project was finding that blocking Fgf signalling inhibited early myf5 and myod induction. I had spent a great deal of time analysing myf5 and myod expression in young but not older adaxial cells of Hh mutants. I was determined to find what midline-derived signals might be involved in regulating the initial Hh-independent MRF expression, and the localisation of Fgf signals made them particularly attractive candidates. Then finding that overexpression of either Fgf4 or Fgf6a could ectopically induce myf5 and myod was the icing on the cake – we knew we were onto something.
KL I think the best was finding that Tbx16 was essential for initial myf5 expression. I found it first with a morpholino; the mutant confirmed it. And then when Fgf4 overexpression could not rescue in the tbx16 mutant background.
And what about the flipside: any moments of frustration or despair?
DO Nothing that really stands out: although there are often such moments in science, it’s just the way it is. For this project it probably revolved around cloning – coming in early to find no colonies on my plates. That’s why it feels so good when experiments go to plan or give an unexpected exciting result. The bitter makes the sweet taste so much better!
KL I was very frustrated not to finish this Fgf story before leaving for China in 2010 due to my (or rather the UK Government’s) problem with visas. At that time, there was a temporary MRC funding hiatus caused by the 2008 financial crisis and the new Government was clamping down on immigrants like me. The result was that I had to leave the country before the end of the year. I finished what experiments I could over Christmas and flew back to China on 31 December 2010. Simon offered me a PhD place to continue the project, but my wife (also a scientist) was back in Wuhan starting her own lab, so in the end I stayed here. I now work in the China Zebrafish Resource Center as a member of the technical staff. My prime duty is to keep the aquarium running properly; no more experiments in my life, which is a great sadness to me.
The bitter makes the sweet taste so much better!
Finally, let’s move outside the lab – what do you like to do in your spare time in London and Wuhan?
DO Family is very important to me, especially with my children being so young (6-10 years). Most of my spare time revolves around taxiing between clubs. However, I have recently got into rock climbing (which the kids do too) and I love tinkering around on my motorbike when given half a chance.
KL I spend most of my spare time with my daughter. She is 4 years old. I teach her English and tell her stories about my life in London. She likes Pocoyo and learns a lot from this cute blue guy.
SH Though I enjoy London’s cultural offerings, my favourite spare time is spent in the hills, either the small ones surrounding our Welsh cottage, or larger ones in places like Argentina; they clear your head with a different kind of excitement and provide space to think. It has never been more important for people to (re-)connect with nature.


 (No Ratings Yet)
(No Ratings Yet)