In Development this week (Volume 137, Issue 15)
Posted by Seema Grewal, on 13 July 2010
Here are the research highlights from the new issue of Development…
TORc1-ing about stem cell differentiation
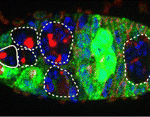 In adult tissues, the tight regulation of stem cell selfrenewal and differentiation maintains tissue homeostasis. In Drosophila ovaries, BMP signalling from the local environment maintains germline stem cells (GSCs) by repressing bam (a differentiation-promoting gene) expression. Now, on p. 2461, Rongwen Xi and co-workers reveal a role for the tumour suppressor tuberous sclerosis complex proteins, TSC1/2, in GSC maintenance. Human TSC1 and TSC2 proteins form a complex that negatively regulates TOR, a conserved kinase involved in cell growth. TOR functions mainly via the TORC1 complex, which activates the protein translation initiator S6K. Disruption of Tsc1 or Tsc2 in Drosophila GSCs, the researchers report, leads to precocious GSC differentiation and loss. Elimination of S6K rescues this phenotype, which implicates TORC1 hyperactivation in the precocious differentiation of Tsc1/2 mutant GSCs. TORC1 hyperactivation also negatively regulates BMP signalling. Thus, suggest the researchers, TSC1/2-TORC1 signalling maintains Drosophila GSCs by controlling both BMP-Bam-dependent and -independent differentiation programs, a role that might be conserved in mammals.
In adult tissues, the tight regulation of stem cell selfrenewal and differentiation maintains tissue homeostasis. In Drosophila ovaries, BMP signalling from the local environment maintains germline stem cells (GSCs) by repressing bam (a differentiation-promoting gene) expression. Now, on p. 2461, Rongwen Xi and co-workers reveal a role for the tumour suppressor tuberous sclerosis complex proteins, TSC1/2, in GSC maintenance. Human TSC1 and TSC2 proteins form a complex that negatively regulates TOR, a conserved kinase involved in cell growth. TOR functions mainly via the TORC1 complex, which activates the protein translation initiator S6K. Disruption of Tsc1 or Tsc2 in Drosophila GSCs, the researchers report, leads to precocious GSC differentiation and loss. Elimination of S6K rescues this phenotype, which implicates TORC1 hyperactivation in the precocious differentiation of Tsc1/2 mutant GSCs. TORC1 hyperactivation also negatively regulates BMP signalling. Thus, suggest the researchers, TSC1/2-TORC1 signalling maintains Drosophila GSCs by controlling both BMP-Bam-dependent and -independent differentiation programs, a role that might be conserved in mammals.
Olfactory neuronal precursors sniffed out
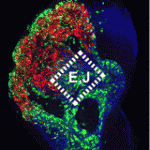 Neuronal precursors in the developing olfactory epithelium (OE) produce olfactory receptor, vomeronasal and gonadotropinreleasing hormone neurons, neuronal classes that are essential for chemosensation, social interactions and reproduction. Now, Anthony-Samuel LaMantia and colleagues characterise two distinct populations of neuronal precursors in the mouse OE that give rise to these neuronal types (see p. 2471). They describe a population of slowly dividing, self-renewing precursors mainly in the lateral OE that express high levels of Meis transcription factors and a population of rapidly dividing neurogenic precursors mainly in the medial OE that express high levels of the Sox2 and Ascl1 transcription factors. The Meis dose in the first population reduces Ascl1 expression and neurogenesis, they report, whereas the Sox2 dose in the second population, which is partly controlled by local Fgf8 signalling, promotes OE neurogenesis by suppressing Meis1 and enhancing Ascl1 expression. These insights into the characteristics of OE neuronal precursors should facilitate the identification of the adult OE neural stem cells that generate olfactory receptor and vomeronasal neurons throughout life.
Neuronal precursors in the developing olfactory epithelium (OE) produce olfactory receptor, vomeronasal and gonadotropinreleasing hormone neurons, neuronal classes that are essential for chemosensation, social interactions and reproduction. Now, Anthony-Samuel LaMantia and colleagues characterise two distinct populations of neuronal precursors in the mouse OE that give rise to these neuronal types (see p. 2471). They describe a population of slowly dividing, self-renewing precursors mainly in the lateral OE that express high levels of Meis transcription factors and a population of rapidly dividing neurogenic precursors mainly in the medial OE that express high levels of the Sox2 and Ascl1 transcription factors. The Meis dose in the first population reduces Ascl1 expression and neurogenesis, they report, whereas the Sox2 dose in the second population, which is partly controlled by local Fgf8 signalling, promotes OE neurogenesis by suppressing Meis1 and enhancing Ascl1 expression. These insights into the characteristics of OE neuronal precursors should facilitate the identification of the adult OE neural stem cells that generate olfactory receptor and vomeronasal neurons throughout life.
Ringing the changes on bivalent gene silencing
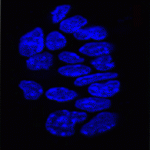 In pluripotent ES cells, key developmental regulators contain ‘bivalent chromatin domains’ – regions that carry epigenetic markers of both repressed and active chromatin, and that assemble RNA polymerase (RNAP) complexes. Thus, these bivalent domains silence genes, but keep them primed for timely activation and are thought to resolve into repressed or active domains upon ES cell differentiation. But are bivalent chromatin domains involved in in vivo development? On p. 2483, Véronique Azuara and colleagues report that these domains operate in the early mouse embryo. They show that several somatic lineage regulators (including Hox factors) retain bivalent chromatin domains in cells that are committed to the extra-embryonic lineage. However, these genes, in contrast to similar genes in pluripotent cells, are not engaged by the Polycomb repressive complex component Ring1B. Instead, these bivalent genes are selectively targeted for Suv39h1-mediated repression through H3K9 methylation, and for RNAP exclusion upon trophoblast lineage commitment. Thus, Ring1B and Suv39h1 play mutually exclusive roles in the establishment of distinct chromatin states during early mouse lineage commitment.
In pluripotent ES cells, key developmental regulators contain ‘bivalent chromatin domains’ – regions that carry epigenetic markers of both repressed and active chromatin, and that assemble RNA polymerase (RNAP) complexes. Thus, these bivalent domains silence genes, but keep them primed for timely activation and are thought to resolve into repressed or active domains upon ES cell differentiation. But are bivalent chromatin domains involved in in vivo development? On p. 2483, Véronique Azuara and colleagues report that these domains operate in the early mouse embryo. They show that several somatic lineage regulators (including Hox factors) retain bivalent chromatin domains in cells that are committed to the extra-embryonic lineage. However, these genes, in contrast to similar genes in pluripotent cells, are not engaged by the Polycomb repressive complex component Ring1B. Instead, these bivalent genes are selectively targeted for Suv39h1-mediated repression through H3K9 methylation, and for RNAP exclusion upon trophoblast lineage commitment. Thus, Ring1B and Suv39h1 play mutually exclusive roles in the establishment of distinct chromatin states during early mouse lineage commitment.
On PAR1 spindle orientation promotes neurogenesis
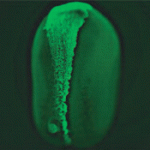 In the developing vertebrate CNS, ‘deep’ cells differentiate into neurons whereas undifferentiated superficial epithelial cells continue to proliferate. The rate of neuronal differentiation depends on the balance between these two cell types, which are generated by asymmetric divisions of the superficial cells. Now, Jeremy Green and co-workers reveal that the conserved polarity protein PAR-1 promotes these asymmetric divisions in the neural plate of Xenopus embryos by controlling spindle orientation (see p. 2501). PAR-1, which is basolaterally localised in epithelia, is required for the differentiation of deep cells. By grafting marked superficial cells that express activated PAR-1 onto untreated embryos, the researchers show that PAR-1 drives the generation of deep cells from the superficial epithelium. Depletion experiments indicate that PAR-1 is normally required for vertically orientating epithelial mitotic spindles, thereby ensuring a sufficient number of asymmetric cleavages. Importantly, the effect of PAR-1 on spindle orientation not only generates deep cells, note the researchers, but also promotes neurogenesis by partitioning these cells away from anti-neurogenic, apically localised atypical protein kinase C.
In the developing vertebrate CNS, ‘deep’ cells differentiate into neurons whereas undifferentiated superficial epithelial cells continue to proliferate. The rate of neuronal differentiation depends on the balance between these two cell types, which are generated by asymmetric divisions of the superficial cells. Now, Jeremy Green and co-workers reveal that the conserved polarity protein PAR-1 promotes these asymmetric divisions in the neural plate of Xenopus embryos by controlling spindle orientation (see p. 2501). PAR-1, which is basolaterally localised in epithelia, is required for the differentiation of deep cells. By grafting marked superficial cells that express activated PAR-1 onto untreated embryos, the researchers show that PAR-1 drives the generation of deep cells from the superficial epithelium. Depletion experiments indicate that PAR-1 is normally required for vertically orientating epithelial mitotic spindles, thereby ensuring a sufficient number of asymmetric cleavages. Importantly, the effect of PAR-1 on spindle orientation not only generates deep cells, note the researchers, but also promotes neurogenesis by partitioning these cells away from anti-neurogenic, apically localised atypical protein kinase C.
Ectodermin damps down Nodal
 During early vertebrate embryogenesis, gradients of the TGFβ-related factor Nodal control embryonic pluripotency and establish the body plan. But how do embryonic cells interpret subtle changes in Nodal signalling? According to Stefano Piccolo and colleagues, the negative intracellular Smad regulator ectodermin (Ecto) determines how mouse embryonic cells read Nodal signals in vivo (see p. 2571). Recent results suggest that the ubiquitin ligase ectodermin acts as an intracellular regulator of TGFβ signalling by monoubiquitylating Smad4, which causes the disassembly of the R-Smad/Smad4 transcriptional complex that mediates TGFβ signalling. Here, the researchers show that ablation of Ecto in trophoblast cells disrupts the balance between stem cell self-renewal and differentiation by increasing their Nodal responsiveness, a result that reveals a new role for Nodal signalling in trophoblast development. In the epiblast, they report, Ecto deficiency shifts mesoderm fates towards node/organiser fates. These and other results suggest that the negative control of Smad activity by ectodermin orchestrates early mouse development by ‘tuning’ the responses of extra-embryonic and embryonic cells to Nodal.
During early vertebrate embryogenesis, gradients of the TGFβ-related factor Nodal control embryonic pluripotency and establish the body plan. But how do embryonic cells interpret subtle changes in Nodal signalling? According to Stefano Piccolo and colleagues, the negative intracellular Smad regulator ectodermin (Ecto) determines how mouse embryonic cells read Nodal signals in vivo (see p. 2571). Recent results suggest that the ubiquitin ligase ectodermin acts as an intracellular regulator of TGFβ signalling by monoubiquitylating Smad4, which causes the disassembly of the R-Smad/Smad4 transcriptional complex that mediates TGFβ signalling. Here, the researchers show that ablation of Ecto in trophoblast cells disrupts the balance between stem cell self-renewal and differentiation by increasing their Nodal responsiveness, a result that reveals a new role for Nodal signalling in trophoblast development. In the epiblast, they report, Ecto deficiency shifts mesoderm fates towards node/organiser fates. These and other results suggest that the negative control of Smad activity by ectodermin orchestrates early mouse development by ‘tuning’ the responses of extra-embryonic and embryonic cells to Nodal.
Polycomb recruitment to DNA: Spps enlisted
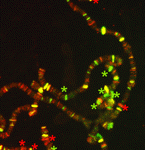 Polycomb group (PcG) protein complexes repress gene expression during the development of higher eukaryotes by binding to Polycomb group response elements (PREs). Little is known about how PcG complexes are recruited to PREs but, on p. 2597, Lesley Brown and Judith Kassis suggest that Spps (Sp1-like factor for Pairing Sensitivesilencing) might be involved in this process in Drosophila. All known Drosophila PREs contain binding sites for Sp1/KLF zinc-finger proteins. The researchers now report that the Sp1/KLF family member Spps binds to Ubx and engrailed PREs, and to polytene chromosomes in a binding pattern that closely matches that of the PcG protein Psc. Spps deletion suppresses ‘pairing-sensitive silencing’, they report, a PRE-associated activity in which somatic-chromosome pairing increases PcG-mediated repression. Spps mutation also enhances the phenotype of pho mutants; the PcG protein Pho is involved in, but not sufficient for, PcG complex recruitment to PREs. Together, these results suggest that Spps works with, or in parallel to, Pho to recruit PcG complexes to PREs.
Polycomb group (PcG) protein complexes repress gene expression during the development of higher eukaryotes by binding to Polycomb group response elements (PREs). Little is known about how PcG complexes are recruited to PREs but, on p. 2597, Lesley Brown and Judith Kassis suggest that Spps (Sp1-like factor for Pairing Sensitivesilencing) might be involved in this process in Drosophila. All known Drosophila PREs contain binding sites for Sp1/KLF zinc-finger proteins. The researchers now report that the Sp1/KLF family member Spps binds to Ubx and engrailed PREs, and to polytene chromosomes in a binding pattern that closely matches that of the PcG protein Psc. Spps deletion suppresses ‘pairing-sensitive silencing’, they report, a PRE-associated activity in which somatic-chromosome pairing increases PcG-mediated repression. Spps mutation also enhances the phenotype of pho mutants; the PcG protein Pho is involved in, but not sufficient for, PcG complex recruitment to PREs. Together, these results suggest that Spps works with, or in parallel to, Pho to recruit PcG complexes to PREs.


 (No Ratings Yet)
(No Ratings Yet)