A Day in the Life of a Killifish Lab
Posted by Andrew Thompson, on 25 September 2022
What is a Killifish?
My name is Andrew Thompson, and I am an assistant professor and principal investigator of the Xtremo-Devo Lab at Western Michigan University. In our lab, we use small, colorful, tropical killifishes (Fig.1) to study how organisms adapt to extreme environments and undergo suspended animation. Killifishes include freshwater fishes in the group of Aplocheiloidei (Cyprinodontiformes) and live in small freshwater pools and streams in the tropics of South and Central America, Africa, Madagascar, India, and Southeast Asia. In fact, it is thought that “killi” is derived from the archaic Dutch word “kilde” meaning small stream or puddle.

A Seasonal Life Cycle
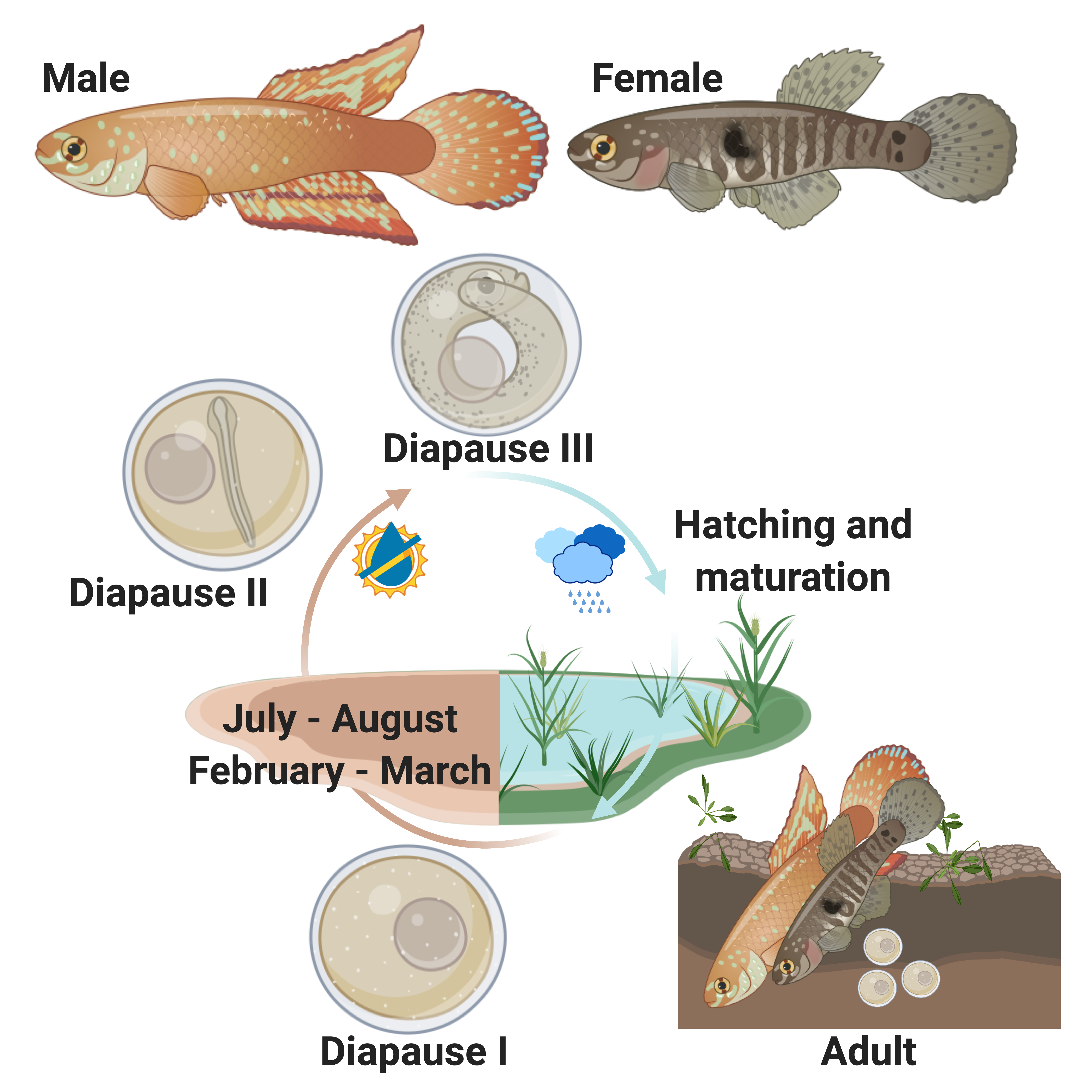
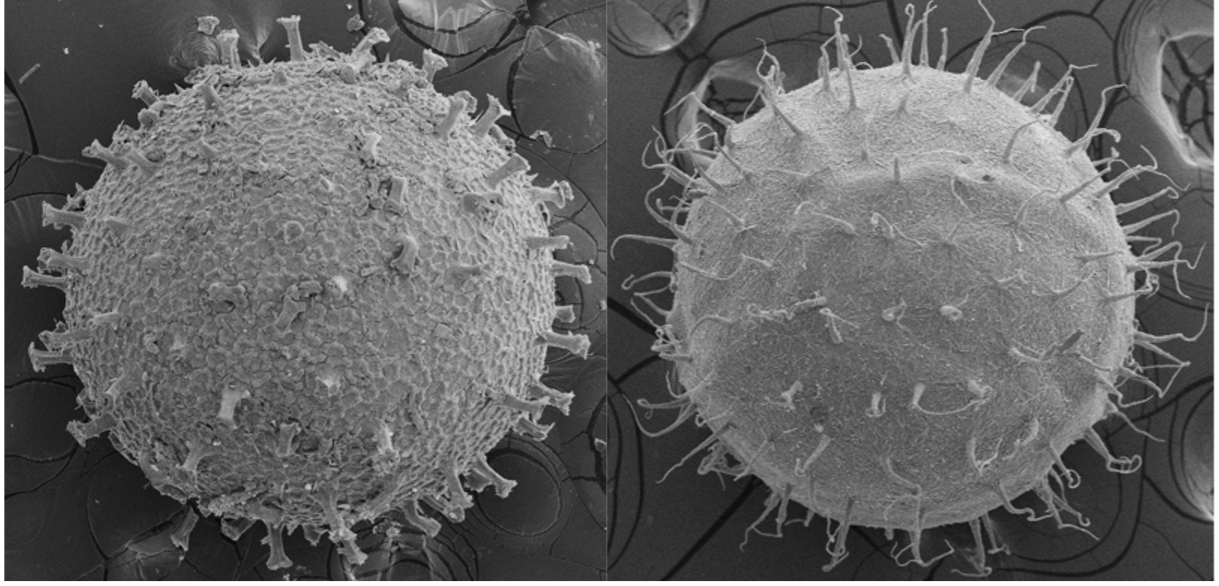
Some killifish species in Africa and South America live in pools that seasonally flood in a wet season and dry out in a dry season (Fig. 2). This can happen once a year, twice a year, or less predictably depending on weather and climate patterns (Costa 2002; Furness 2015). Unfortunately, for these killifish, the adults living in seasonal or annual pools are doomed to die in the dry season and the desiccation of their habitat. But as Dr. Ian Malcom from Jurassic Park might say, “life, uh, finds a way.” And killifish did find a way. Around the time dinosaurs were going extinct, Aplocheiloid Killifish were beginning to diversify (Thompson et al. 2021). They evolved superpowers that allowed some species to survive in these temporary habitats. More specifically, these “fish-out-of-water” bury their eggs in the soil that have tough specialized egg envelopes (Fig. 3) that keep water in and prevent the embryos from drying out during the dry season. These eggs can be covered in some amazing structures like filaments to bind them to the substrate, and even mushroom-like (Fig 3A) or corkscrew-like projections (Fig. 3B, Thompson et al. 2017a).
Dormancy and Diapause
But what about timing? These annual or seasonal killifishes cannot just grow and hatch like most fish when they get to a certain time in development. If they grow too fast or hatch at the wrong time, they will die upon hatching if the pools have not refilled or already dried up. Annual killifishes have a strategy that is unique among vertebrates in that they can arrest their development up to three different times as embryos buried in the soil (Fig. 2, Wourms 1972a; Wourms 1972b; Wourms 1972c). This arrested development is known as diapause, and few vertebrates are capable of this type of dormancy. During diapause, growth stops, cells stop dividing, and metabolic rate is greatly reduced. Killifish diapause can occur at three specific embryonic stages and can be skipped depending on environmental conditions such as temperature (Podrabsky et al. 2010). The first diapause occurs very early after fertilization when the embryo consists of stem cells (Fig.2, Wourms 1972b; Wourms 1972a). The second diapause stage occurs when the embryo is starting to develop organs (Fig. 2, Wourms 1972a; Wourms 1972c). The third diapause is used to delay hatching and begins after organs have fully developed (post organogenesis, Fig. 2, Wourms, 1972a, 1972c). Interestingly, non-annual killifishes can also delay hatching, especially if they are incubated out of water, but this might not represent a dormant or diapause phenotoype (Wourms 1972c; Varela-Lasheras and van Dooren 2014; Furness 2015; Thompson et al. 2017b).
Environmentally-Cued Hatching
Annual killifishes are the only vertebrates to stop their development after organogenesis, and this unique type of suspended animation could inform research on how vertebrate animals survive stasis with fully-developed, complex organ systems. Humans have long fantasized about “hypersleep”, a key plot point in science fiction films used to travel into deep space, save energy, stop aging, and survive in harsh environments, but killifish have been practicing this technique for millions of years. Killifish are able to use Diapause III to control hatching, remaining dormant at this stage until their habitat floods, triggering them to hatch. Hatching is a process that most animals and all vertebrates must complete as part of their development. Aquatic vertebrates like fish and frogs hatch by secreting enzymes that break down the egg envelope or zona pellucida and allow the transition from an embryo to a free-living larvae (Urch and Hedrick 1981; Yamagami 1981; Yasumasu et al. 1992; Cohen et al. 2018). While you may think of human hatching as a birthday, humans and other mammalian embryos hatch much earlier in development, during the blastocyst stages when the embryo and/or the uterus secrete enzymes that break down the zona pellucida so implantation can occur (Yamazaki et al. 1994; O’Sullivan et al. 2002; Syrkasheva et al. 2017; Leonavicius et al. 2018).
Eco-Evo-Devo
Our lab wants to figure out how animals integrate genetic and environmental cues to control progression of development in the face of changing environments. Killifish offer a unique opportunity as a research model to study suspended animation and the environmental control of hatching in a vertebrate system. While we understand the adaptive nature of dormancy and the enzymatic component of hatching, the genetic regulation of these developmental transitions or lack thereof via diapause, are relatively unknown. The study of evolutionary developmental biology or “Evo-Devo” has provided unprecedented insights into how myriad organisms turn genotype in to phenotype, but our killifish model system allows for an “Eco-Evo-Devo” approach, exploring the integration of environmental cues with the underlying genetic regulatory signals to create phenotype.
The Rio Pearlfish
We have been developing the Rio Pearlfish (Fig. 4) as a tractable annual killifish model to study both the suspended animation and environmental control of hatching (Thompson and Ortí 2016; Thompson et al. 2022). Rio Pearlfish are quite happy in small containers that mimic their small puddles in the wild. They breed very easily in the lab in sand (Video 1) and eggs can be sifted out and used for studies that manipulate both the genome and environment of developing and diapausing eggs. For example, eggs can be incubated in humid environments outside of water, and we often place them in small plastic containers on top of moist peat moss to keep them damp and mimic seasonal desiccation (Fig. 5) Once our Pearlfish reach diapause III, we simply add water to halt dormancy, induce hatching, and begin the next generation (Fig. 6 ). Rio Pearlfish evolved a seasonal life history and the associated diapauses independently from other annual killifish species in Africa and South America (Thompson et al., 2021). We have sequenced the genome of the Rio Pearlfish, identified the hatching gland (Thompson et al. 2022), and characterized changes in gene expression between diapause III and hatched fish, many of which are convergent during dormancy across metazoans (Thompson and Ortí 2016). Thus, the Rio Pearlfish will be an invaluable laboratory model in our lab as we explore convergent adaptations to extreme environments..



A Future of Killifish
Overall, we aim to use our investigations into diapause and environmentally-cued hatching in the Rio Pearlfish and other killifishes to learn more about how animals adapt to stressful environments in a changing world. This is especially important in the light of pervasive human-induced environmental change. We also aim to strengthen the use of killifishes as biomedical models since they can slow growth, development, and metabolic rate. Perhaps, someday, killifish suspended animation could inform research on human diseases involving growth retardation, metabolic disorders, or abnormal cellular division.
We are currently recuiting Ph.D. and Master’s students, so if you are interested in joining our team or hearing more about our research, please contact us and check out our website here.
References:
Cohen, K. L., Piacentino, M. L., & Warkentin, K. M. (2019). Two types of hatching gland cells facilitate escape-hatching at different developmental stages in red-eyed treefrogs, Agalychnis callidryas (Anura: Phyllomedusidae). Biological Journal of the Linnean Society, 126(4), 751-767.
Costa, W. J. E. M. (2002). The neotropical seasonal fish genus Nematolebias (Cyprinodontiformes: Rivulidae: Cynolebiatinae): taxonomic revision with description of a new species. Ichthyological Exploration of Freshwaters, 13(1), 41-52.
Furness, A. I. (2016). The evolution of an annual life cycle in killifish: adaptation to ephemeral aquatic environments through embryonic diapause. Biological Reviews, 91(3), 796-812.
Leonavicius, K., Royer, C., Preece, C., Davies, B., Biggins, J. S., & Srinivas, S. (2018). Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay. Proceedings of the National Academy of Sciences, 115(41), 10375-10380.
O’Sullivan, C. M., Liu, S. Y., Karpinka, J. B., & Rancourt, D. E. (2002). Embryonic hatching enzyme strypsin/ISP1 is expressed with ISP2 in endometrial glands during implantation. Molecular Reproduction and Development: Incorporating Gamete Research, 62(3), 328-334.
Podrabsky, J. E., Garrett, I. D., & Kohl, Z. F. (2010). Alternative developmental pathways associated with diapause regulated by temperature and maternal influences in embryos of the annual killifish Austrofundulus limnaeus. Journal of Experimental Biology, 213(19), 3280-3288.
Shafei, R. A., Syrkasheva, A. G., Romanov, A. Y., Makarova, N. P., Dolgushina, N. V., & Semenova, M. L. (2017). Blastocyst hatching in humans. Russian Journal of Developmental Biology, 48(1), 5-15.
Thompson, A. W., Black, A. C., Huang, Y., Shi, Q., Furness, A. I., Braasch, I., … & Ortí, G. (2021). Deterministic shifts in molecular evolution correlate with convergence to annualism in killifishes. BioRxiv.
Thompson, A. W., Furness, A. I., Stone, C., Rade, C. M., & Ortí, G. (2017). Microanatomical diversification of the zona pellucida in aplochelioid killifishes. Journal of Fish Biology, 91(1), 126-143.
Thompson, A. W., Hayes, A., Podrabsky, J. E., & Ortí, G. (2017). Gene expression during delayed hatching in fish-out-of-water. Ecological Genetics and Genomics, 3, 52-59.
Thompson, A. W., & Ortí, G. (2016). Annual killifish transcriptomics and candidate genes for metazoan diapause. Molecular biology and evolution, 33(9), 2391-2395.
Thompson, A. W., Wojtas, H., Davoll, M., & Braasch, I. (2022). Genome of the Rio Pearlfish (Nematolebias whitei), a bi-annual killifish model for Eco-Evo-Devo in extreme environments. G3, 12(4), jkac045.
Urch, U. A., & Hedrick, J. L. (1981). Isolation and characterization of the hatching enzyme from the amphibian, Xenopus laevis. Archives of Biochemistry and Biophysics, 206(2), 424-431.
Varela-Lasheras, I., & Van Dooren, T. J. (2014). Desiccation plasticity in the embryonic life histories of non-annual rivulid species. EvoDevo, 5(1), 1-11.
Wourms, J. P. (1972). Developmental biology of annual fishes. I. Stages in the normal development of Austrofundulus myersi Dahl. Journal of Experimental Zoology, 182(2), 143-167.
Wourms, J. P. (1972). The developmental biology of annual fishes. II. Naturally occurring dispersion and reaggregation of blastomeres during the development of annual fish eggs. Journal of Experimental Zoology, 182(2), 169-200.
Wourms, J. P. (1972). The developmental biology of annual fishes. III. Pre‐embryonic and embryonic diapause of variable duration in the eggs of annual fishes. Journal of Experimental Zoology, 182(3), 389-414.
Yamagami, K. (1981). Mechanisms of hatching in fish: secretion of hatching enzyme and enzymatic choriolysis. American Zoologist, 21(2), 459-471.
Yamazaki, K., Suzuki, R., Hojo, E., Kondo, S., Kato, Y., Kamioka, K., … & Sawada, H. (1994). Trypsin‐like Hatching Enzyme of Mouse Blastocysts: Evidence for Its Participation in Hatching Process before Zona Shedding of Embryos 6: (embryo/hatching enzyme/protease/trypsin/strypsin). Development, growth & differentiation, 36(2), 149-154.
Yasumasu, S., Yamada, K., Akasaka, K., Mitsunaga, K., Iuchi, I., Shimada, H., & Yamagami, K. (1992). Isolation of cDNAs for LCE and HCE, two constituent proteases of the hatching enzyme of Oryzias latipes, and concurrent expression of their mRNAs during development. Developmental biology, 153(2), 250-258.


 (1 votes)
(1 votes)
I am looking for a killifish lab activity that I could tie into concepts taught in AP Environmental Science, would anyone happen to know of one that exists I could use or use as a model to create my own from scratch?