A day in the life of two Sea Star labs
Posted by Margherita Perillo, on 13 November 2023
What comes to mind when I say, “sea star”? For me, I think of easily accessible eggs that we can fertilized in vitro to make completely clear larvae that grow in a 6-well dish. Ah yes, I guess you were also thinking about snorkeling in a transparent ocean!
My name is Margherita Perillo and I am a Research Scientist at the MBL in beautiful Woods Hole right in Cape Cod. My research focuses mostly on understanding tissue and organ morphogenesis: How do individual cells group together to form complex organs? The system I chose to establish to investigate this question is the sea star Patiria miniata larva. In this short article, together with Zak Swartz (Assistant Scientist at MBL who also works with sea stars) and Jamie MacKinnon (Research Assistant from the Swartz Lab), we explain why we love this research animal.
Who works at the MBL?
The Marine Biological Laboratory is a vibrant year-round institute for research and teaching affiliated with the University of Chicago (Fig. 1). You may know us for our summer season, when we host advanced research training courses including the famous Embryology and Physiology courses, as well as visiting scientists and students from around the world, reaching a campus population of around 1,200 people. But throughout the year, MBL is home to over 30 resident faculty and laboratories across three departments, including Ecosystems Center, the Josephine Bay Paul Center for Comparative Molecular Biology and Evolution, and the Eugene Bell Center for Regenerative Biology and Tissue Engineering. Our research community spans different length scales and disciplines, from biomedical cell biology to ecosystem-level interactions. In addition, the MBL offers immersive undergraduate courses, including the Semester in Environmental Science and the new Semester in Biological Discovery, and a brand new Ph.D. program in conjunction with the University of Chicago.

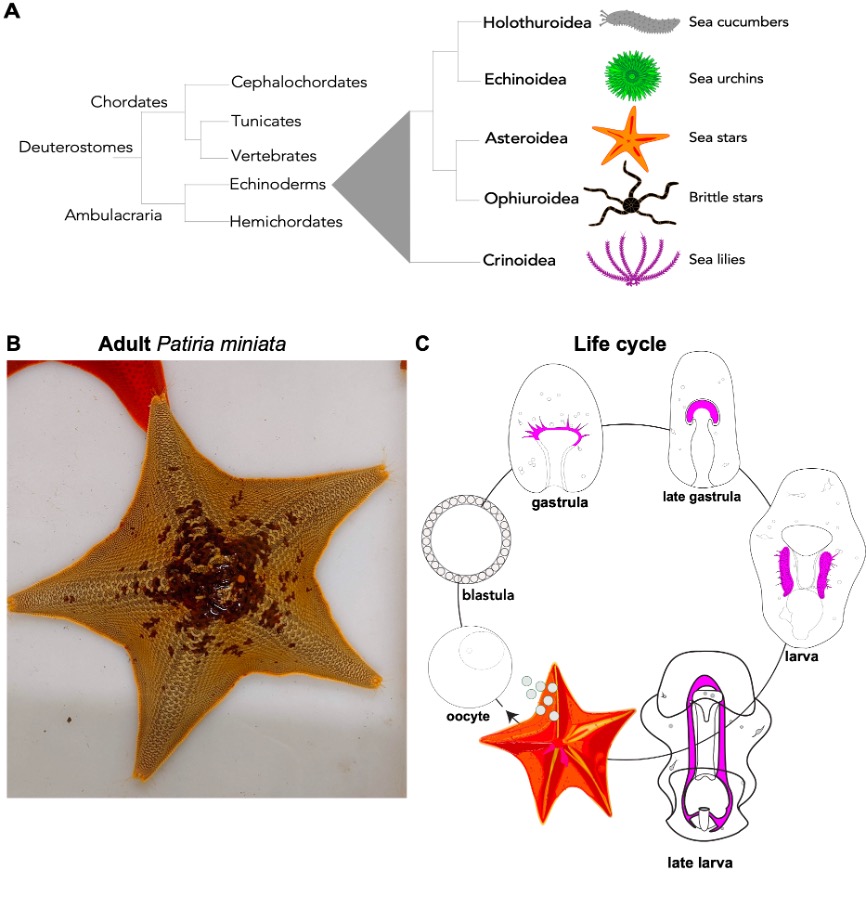
Patiria miniata in the wild
Sea stars are echinoderms, a group of bilaterian animals that includes sea urchins, sea stars, sea lilies, brittle stars, and sea cucumbers. Because of their close relationship with vertebrates, these animals are great models to ask biomedical questions, as the basic cellular and developmental mechanisms that we study in sea stars are conserved in vertebrates (Fig. 2A). The sea star Patiria miniata (Fig. 2B) can be found all along the Pacific Coast, from Alaska to Mexico in deep and shallow waters 1,2. We get our animals from divers in California who ship us sea stars that we keep in big tanks in the MBL Marine Resource Center. Here a team of sea star experts takes care of them to make sure they enjoy their stay and have the best possible accommodations in Cape Cod.
Life cycle: Females and male adult sea stars live in groups and when the season is right, they release their gametes out in the ocean where fertilization happens (Fig. 2C). There are gametes are in each arm and if we are lucky we find a female with six arms -extra ovaries for us! Embryos and larvae of P. miniata go through gastrulation and transform into planktonic larvae. After a few months, the larvae undergo metamorphosis to create a tiny, juvenile sea star. A remarkable feature of sea stars (and all other echinoderms) is that while their adult body has a pentameric plan, their larvae are bilateral, meaning that if we draw a line in the center of the larva there is a left and a right side, like us!
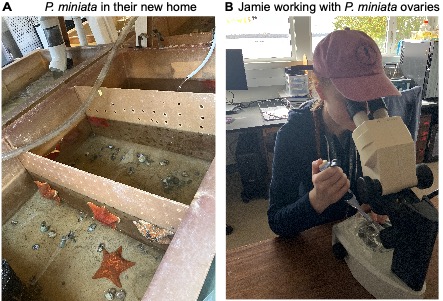
Patiria miniata in the laboratory
One of the best parts about working with sea stars is that they are incredibly easy to culture and bring through metamorphosis. A normal week in the lab begins with a trip to the Marine Resources Center (MRC) to visit our adult sea stars, check their health, and collect gonads (Fig. 3A). We carefully make a 1mm ventral incision and extract a piece of ovary; these pieces are cultured in antibiotic-treated seawater and safely kept ex-vivo for weeks at 15°C3,4.
When we need to expand our larval cultures, we use a dissecting needle to tease open the ovary, remove any eggs we need for the day, and add hormone to induce maturation (Fig. 3B). After fertilization, the early-stage cell divisions will happen in just a few hours. Two days later they will have developed into swimming larvae which can be transferred into 500mL boxes and fed with a red and green algal cocktail. If we change the water biweekly and continue this feeding pattern, we can observe bipinnaria larvae beginning to metamorphose within a few months. At this time we begin to feed larvae small pieces of Aquanix kelp flakes, containing spirulina, and several sources of protein. The juvenile sea stars are very low maintenance and continue to grow larger and more motile day by day!
P. miniata, an emerging system to understand organ morphogenesis
Our body is composed of many organs with diverse functions. What do they all have in common? Well, virtually all organs derive from epithelial tubes. During organogenesis these simple tubes grow, branch and elongate to make complex organs like lungs, kidneys, heart, pancreas and more. If this first step of making a tube goes wrong the embryo will develop with major birth defects with one or more organs that are shorter, have the wrong orientation in the embryo and do not function properly5-7.
Because of the fundamental role that epithelial tubes have in building our organs a key question is: What are the mechanisms that drive proper outgrowth and elongation of epithelial tube? And what can be a good model to address this question?
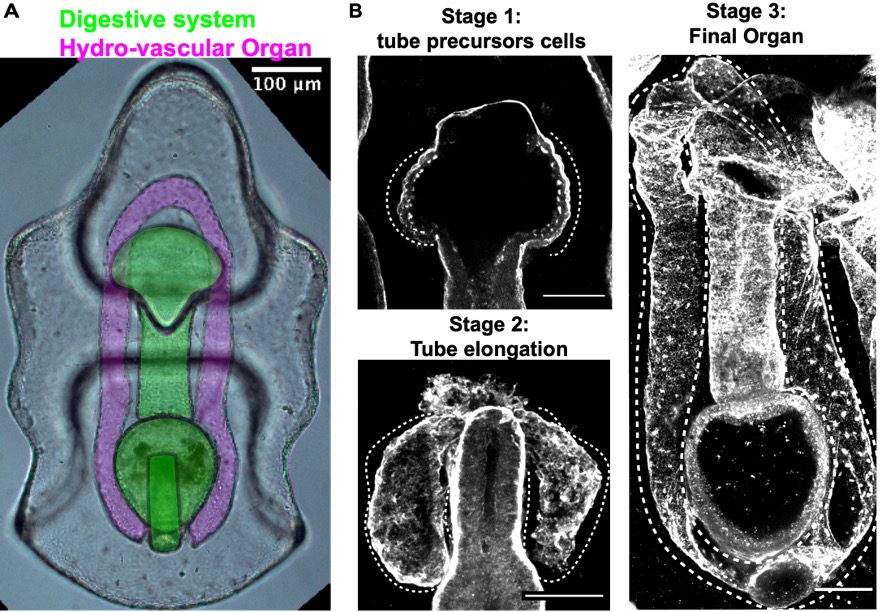
While vertebrates have many, complex and highly branched organs all tightly packed together, the sea star larva has only two simple and optically clear organs: a digestive system and the hydro-vascular organ, (HVO) (Fig. 4). In my recent work, I develop two important tools that allowed us to use this new system to study how tubes form: long-term live imaging (to look at cell movements) and I set up the first CRISPR Cas9 protocols for a sea star (to perturb gene function)8-10.
The HVO is the perfect epithelial tube: we found that it starts as a sheet of cells that bud off the digestive system (stage 1) to form two parallel tubes (stage 2) that elongate, make one branch and eventually fuse to form a looped organ (stage 3). HVO functions might be related to larval buoyancy in the water column11 and I’m investigating if this is its only function.
We used the HVO as a model to define aspects of tube morphogenesis that were still poorly defined, like for instance: What drives tube elongation? We found that the FGF pathway is a major driver of tube outgrowth and that this pathway also controls branch point formation through the transcriptional factor Six1/2. Using live imaging we investigated the mechanics of tube elongation and found that cells of the growing tube actively migrate and at the same time divide to allow for tube extension and expansion. This is relevant from a biomedical perspective, as these steps are conserved with mammals 8.
Sea stars for fundamental reproductive biology
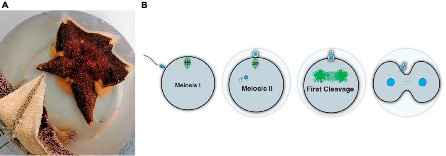
In the lab of Zak Swartz, we work with sea stars to explore fundamental reproductive processes from a cell biological perspective. In contrast to mammals, which undergo reproductive aging and have limited fecundity, the sea star produces millions of new oocytes throughout its (30 year+) lifespan through adult oogenesis (Figure 5A). This is a practical advantage, as having such abundant access to ovary tissue and oocytes lowers the barriers to doing our experiments. But it also fascinating biology: how do sea stars manage to continuously produce so many oocytes whereas humans are born with a limited set? Periklis Paganos is leading a project that uses single-cell genomics to define the cell type repertoire that drives this reproductive longevity, and cell biological approaches to understand how these cells interact with each other. Our goal is to define the signaling interactions and cellular states that support a long reproductive lifespan, which we hope will help inform human fertility treatments.
Another special aspect of working with sea stars is their status as ecologically important animals. As predators and keystone species, they have an outsized impact on food webs. Like many other marine invertebrates, sea stars release their eggs directly into the seawater, with minimal protection against any fluctuations in the environment. Yet, they are fertilized and must accurately perform meiotic and mitotic processes to form an embryo under these conditions (Figure 5B). Jamie MacKinnon is asking how resilient sea star reproduction is to climate change, including variables such as temperature. By comparing eggs from different species, we aim to identify predictive measures for how marine eggs and early embryos will respond to extreme climate fluctuations. We are also working developing new genetic tools for sea stars, an effort led by Akshay Kane in our lab, and Nat Clarke at MIT, that we hope will make sea stars and other echinoderms more accessible for the research community – stay tuned!
Patiria miniata combines a biomedically relevant phylogenetic position, genetic tools for functional analysis and a lot of oocytes and embryos available year-round -we are excited to learn more from these model organisms in the future.
This post was co-written by Margherita Perillo, Zak Swartz and Jamie MacKinnon
References
1 Ebert, T. A. Life-History Analysis of Asterinid Starfishes. The Biological Bulletin 241, 231-242, doi:10.1086/716913 (2021).
2 Morris, R. H., Abbott, D. P. & Haderlie, E. C. Intertidal invertebrates of California. Vol. 200 (Stanford University Press Stanford, 1980).
3 Swartz, S. Z. et al. Quiescent cells actively replenish CENP-A nucleosomes to maintain centromere identity and proliferative potential. bioRxiv, 433391 (2018).
4 Pal, D., Visconti, F., Sepúlveda-Ramírez, S. P., Swartz, S. Z. & Shuster, C. B. Use of echinoderm gametes and early embryos for studying meiosis and mitosis. Mitosis: Methods and Protocols, 1-17 (2022).
5 Ely, D. M. & Driscoll, A. K. Infant Mortality in the United States, 2020: Data From the Period Linked Birth/Infant Death File. Natl Vital Stat Rep 71, 1-18 (2022).
6 Baldwin, D. & Yadav, D. in StatPearls (StatPearls Publishing
Copyright © 2023, StatPearls Publishing LLC., 2023).
7 Eitler, K., Bibok, A. & Telkes, G. Situs Inversus Totalis: A Clinical Review. Int J Gen Med 15, 2437-2449, doi:10.2147/ijgm.S295444 (2022).
8 Perillo, M., Swartz, S. Z., Pieplow, C. & Wessel, G. M. Molecular mechanisms of tubulogenesis revealed in the sea star hydro-vascular organ. Nature Communications 14, 2402, doi:10.1038/s41467-023-37947-2 (2023).
9 Oulhen, N., Pieplow, C., Perillo, M., Gregory, P. & Wessel, G. M. Optimizing CRISPR/Cas9-based gene manipulation in echinoderms. Dev Biol 490, 117-124, doi:10.1016/j.ydbio.2022.07.008 (2022).
10 Perillo, M., Swartz, S. Z. & Wessel, G. M. A conserved node in the regulation of Vasa between an induced and an inherited program of primordial germ cell specification. Dev Biol 482, 28-33, doi:10.1016/j.ydbio.2021.11.007 (2022).
11 Potts, W. T. The physiological function of the coelom in starfish larvae and its evolutionary implications. Physiol Biochem Zool 76, 771-775, doi:10.1086/381463 (2003).


 (3 votes)
(3 votes)
Hello Margherita,
My name is Kelly Hardison and I work in the Prepared Microscope Slides department at Carolina Biological Supply Company in Burlington, N.C. Our company provides science teaching materials to schools and universities worldwide. I would like to talk to you about your sea star cultures. If you could get in touch with me at your convenience, I would really appreciate it. Thank you for your time, and I hope to hear from you.
Sincerely,
Kelly