A Day in the Life of…a Xenopus lab
Posted by Gary McDowell, on 2 October 2013
I am Gary McDowell, a postdoctoral researcher at Tufts Centre for Regenerative Biology and Developmental Biology. I have just finished a postdoctoral position in a mass spectrometry lab at Boston Children’s Hospital/Harvard Medical School and I was the only frog researcher in the group. I earned my “frog stripes” studying for my PhD in the lab of Anna Philpott at the University of Cambridge, working on the stability of a particular transcription factor that regulated neurogenesis. Recently, however, I have been trying to look at many peptides and proteins across many different developmental stages by proteomics, as well as doing in vivo experiments to validate proteomic findings.
Xenopus laevis, the organism I primarily work with, is a claw-toed frog from Southern Africa that lays many large eggs, traditionally used for embryology but also for biochemical studies, as extracts can be made from eggs and embryos to provide active cytoplasm in a test tube, that can undergo cell cycles and be used to study many processes, such as protein ubiquitylation and degradation. The eggs and embryos are also easily subjected to microinjection in a highly targeted manner – for example, injecting into one of the two cells formed after the first cleavage means that only one half of the embryo is treated, as cells largely do not move between the left and right sides of the embryo formed at this point. This gives a great internal control for scoring phenotypic changes. The fate map of cells in the early embryo is well-characterised, so that injections into one of four, eight, even 32 cells can be used to target particular germ layers, organs and tissues.
Xenopus are distributed around the world as they used to be used as a pregnancy test in hospitals – female frogs would be injected with urine from women thought to be pregnant and if the frog began to lay, it meant hormones marking pregnancy were present in the urine! This worldwide distribution has led (I believe somewhat unfairly) to Xenopus being blamed for the spread of the devastating chytrid fungus. Xenopus can carry, but do not die of this fungus; but as the fungus can also be carried on the wings of birds, Xenopus may simply be the subject of bad publicity.
Xenopus is an excellent organism for biochemical experiments. Unique among my colleagues in the mass spectrometry lab, I am not limited by the amount of material I require for mass spectrometry experiments. There are neither endless dissections of multiple mice to harvest enough tissue, nor continuous growing of cells in culture to produce the literally tens (sometimes hundreds, for post-translational modification studies!) of litres of cultured cells required. I simply need a few eggs or embryos to get more than enough protein to work with, as early Xenopus laevis embryos are so large and protein-rich. This applies to many biochemical manipulations in Xenopus – the amount of material required for making extracts to study biochemical processes, for Western blotting or RNA-Seq is a small fraction of the material that is easily available from a single frog. To do in vivo follow-up, there are multiple strategies for embryo micromanipulation, explant culture and microinjection of mRNA (for protein overexpression) or morpholinos (small synthetic oligonucleotide mimics that prevent target mRNA translation) for phenotypic analysis, including in situ hybridisation and immunohistochemistry.
One disadvantage is that Xenopus has not been sequenced to the level of many other organisms, which means that there is not yet a great protein database publicly available for searching mass spectrometry data. What is great about mass spectrometry data, though, is that it is “futureproofed”: the same data can be searched again and again using new and improved databases as they become available. This lack of genetic information means that there are also fewer mutant frog lines available compared to other model organisms – a deficiency which is currently being addressed by the Xenopus community.
The frogs that I use are members of the large colony at Harvard Medical School, provided by Marc Kirschner. They usually live in a very large facility, with huge tanks swarming with lively frogs. Every so often, however, some are moved down to the satellite facility next to the frog lab (PICTURE 1). Here they are housed in smaller tanks, and are fed every day. The room is windowless, with a scheduled light cycle so that they still experience “day” and “night”. They are checked on regularly to make sure they are not stressed, and have a continuous aquatic system providing a flow of water suited to their requirements. Feeding is carried out on a daily basis. Several labs use the same frog room so sometimes it’s a chance to catch up on what’s going on and what different people are doing – as the focus of the different labs is quite varied.

Xenopus laevis and their embryos tend to be happier in cooler temperatures; frog labs tend to be held at 18 ˚C which keeps everyone very dynamic (but is unfortunate for those of us who wish to cast our own SDS-PAGE gels). However in the current facility I use, there is a room set aside for frog work which is kept cooler – so unfortunately it can be a cold, windowless affair for long days in the frog room!
My Day in the Frog Lab
4 pm, the day before:
My day actually begins the evening before my planned work – to get the female frogs to lay, they need to be primed with human chorionic gonadotrophin (which nowadays we obtain in a purified form, and not from pregnant women).
9.30 am
After being primed, the next morning eggs can be seen gradually falling from the frog’s cloaca (frogs have only one exit for eggs, urine, and faeces PICTURE 2).

To collect a large amount of eggs at once, however, I have to carry out a procedure rather alarmingly called “squeezing”. Actually, it’s more like a frog massage – I pick up the female and gently stroke her back, which massages out the eggs that fill a large proportion of her body cavity. The eggs are caught in a petri dish of salty solution and fertilised in vitro by the addition of sperm, which is spread over the eggs and then induced to enter the egg by flooding of low-salt solution. The fertilisation of the eggs hopefully results in a visible transformation – eggs are randomly oriented with respect to their pigmented animal hemisphere (PICTURE 3) until fertilisation induces microtubule rearrangement and rotation of the cortex so that the embryos are all oriented with their animal hemispheres pointed skywards (PICTURE 4) (the reason for this is not clearly known, although it is possible that in the murky waters which Xenopus naturally inhabit, dark camouflage may hide the eggs from predators swimming or moving above). One further preparation is required before my work can begin – the removal of the jelly coats that surround the embryos. These thick coats protect the embryos, but will prevent me from microinjecting them or easily lysing them for sample preparation, so they need to go. This requires a brief treatment in cysteine solution, which breaks down the coats and releases the embryos in a more easily manipulated (but also more easily ruptured) form.


The embryos I’ve prepared today are going to be used for two experiments: for the first, I will simply take a few embryos at different timepoints and snap-freeze them in liquid nitrogen. These will then by lysed, and the protein-rich material within will be digested with the protease trypsin, and analysed by mass spectrometry, after the digested peptides are sorted by liquid chromatography. This will give me information about the protein content of the embryos at different stages of development.
The other experiment I am carrying out is a much more common protocol in the Xenopus field: microinjection of morpholinos. Morpholinos are small synthetic oligonucleotide-like molecules – so-called because they are made using morpholine rings instead of ribose-based sugar rings – that can block mRNA translation either altogether at the point of initiation, or by blocking splicing events. The morpholinos I’m injecting are targets identified from a proteomic screen using differentiating stem cells and our hope is to use Xenopus as an in vivo tool to model the effects of blocking the translation of these targets into protein, and to look at what happens. Morpholinos can be tricky – they are prone to precipitating out of solution at temperatures like that maintained in the frog room! Microinjection itself is a very zen art, that requires great patience and strength of will. Individual embryos are injected using a calibrated glass needle connected to a high-pressure air supply and controlled by a micro-manipulator (PICTURES 5, 6). If the embryos are good, they will be like perky beach balls and the needle will press against the embryo surface, which pops back smartly as the needle enters. An all-too-common problem with novice injectors is breaking the needle, which is fragile. If the embryos are not quite so healthy, they may require some more force to penetrate the surface, resulting in needle breakage.

4 pm
At the end, a nice dish of injected embryos was left to develop. I come back later in the afternoon to move the embryos out of the injection dishes, clear out any dead embryos (and today there are not many – just some eggs that clearly were not fertilised – hooray!) and place the embryos in new dishes with fresh buffer.
These are then left to continue development and to be scored for any possible phenotypic changes. Different concentrations of morpholino are injected to look for effects on development and to see whether our targets are having any effect in vivo after being identified by proteomic screening.
The advantage to using Xenopus for this in vivo approach is that they can be used as a screen for large numbers of candidates – provided that you have a hardy frog researcher ready to inject! The embryos are large and easy to manipulate and most importantly they develop very quickly. You can think of an idea at the weekend, start the experiment on Monday and by the end of the week have an answer, far faster than some other vertebrate model systems. And whilst Xenopus may not be understood to be a great model system for diseases in humans, they are a great tool for understanding the mechanisms of these diseases (for a discussion on this topic and why Xenopus is such a useful tool for those of us using it, see Sive, H. (2011) Disease Models and Mechanisms 4, 137-138 ). Certainly as someone who trained initially as a chemist, the discovery of an organism that provides such great in vitro and in vivo tools has revolutionised the way I do science now and hopefully for the years to come.
 This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.
This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.


 (11 votes)
(11 votes)
I was wondering… what do lab frogs eat?
So usually just a feed that’s a bit like fish food pellets. Although if they’re very lucky, they get maggots, which they really love!
Great post! I am new to this system and I was wondering what type of mold you use to align your embryos for microinjection. They look so perfectly aligned!
We use 3% agarose and put the backside of a ping pong paddle coating. You have to try the right size of the knob
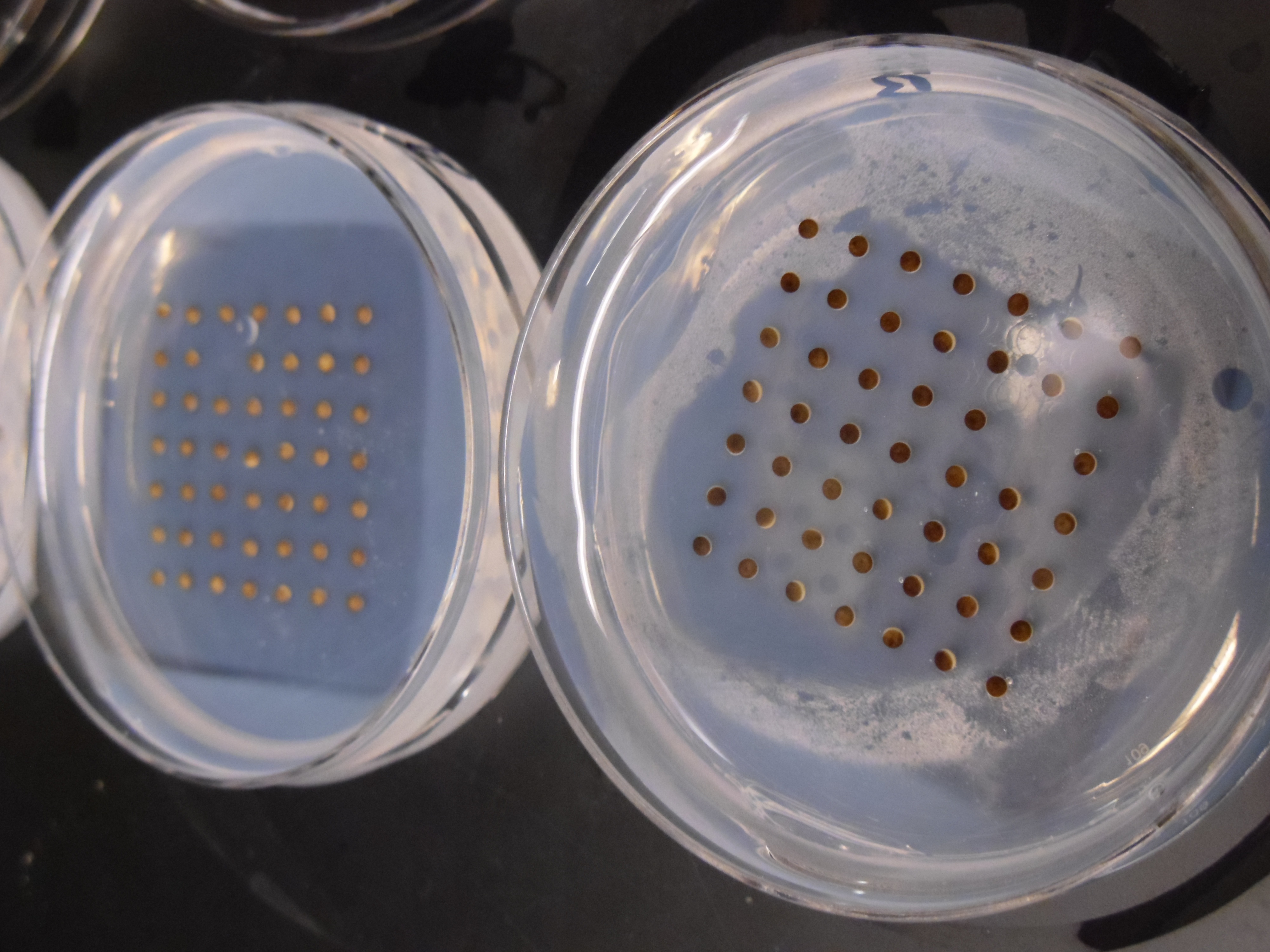
Hi- the two dishes show eggs that are very different colors. Can you comment on why they are different colors and what that means for your experiments?
Hi,
They are from two different females – the pigmentation varies from female to female. It doesn’t generally mean much, although general consensus is that darker, and especially even and not patchy/mottled pigmentation, tend to fertilize better.
Hi,
I’m a PhD student whose focus is more on ecology than biochemistry. But I have a quick question for you since I have recently started using Xenopus laevis embryos. You may not be able to answer this, but it’s worth a shot.
During early stage embryonic development, do X. laevis embryos exhibit similar behavior to that of other frog species (e.g. Rana temporaria)? Does the jelly capsule absorb water from the surroundings causing the jelly to swell, or is there very little change in the size of the embryos as development continues?
Hi,
This is a good question, and maybe a Rana person would be better equipped to answer it/compare. The embryo does increase dramatically in size before ingesting any food, and this is largely due to water uptake. I don’t believe this is due to the jelly itself, but in the lab we always remove the jelly coat reasonably early on in embryo development so I can’t be sure.
Hi
I have just started to work with xenopus laevis but i am facing difficulties in microinjection.can you please guide me for trimming the needle to get appropriate drop size.
Hi,
Try this for a start:
http://www.protocol-online.org/forums/uploads/monthly_04_2011/msg-6470-020563500%201302033009.ipb
I use a 10 nanolitre drop size – it’ll depend on your own microscope magnification how you measure it. Key to this is getting an eyepiece which has a reticle in it so you can judge the size of the drop, and break the needle/adjust pressure and injection time accordingly.