A regeneration retrospective: a budding tale
Posted by Alex Eve, on 6 September 2023
This post is part of the regeneration retrospective series.
Epimorphic regeneration, the process of replacing lost appendages, is probably the most impressive example of tetrapod vertebrate regeneration. Vertebrate appendages, such as limbs and tails, are composed of many different cell types from different germ layers (including, for example, neurons, skin, muscle, bone and endothelium), which require the complex integration of positional information and patterning to regenerate functional structures (Cox et al., 2019). Amphibians are probably the best-known executors of epimorphic regeneration, with the pink, smiley-faced axolotl intriguing scientists since the 16th century (Joven et al., 2019). Even five centuries later, researchers are continuing to study amphibians to understand epimorphic regeneration. While studies have revealed the importance of blastema and a handful of signalling factors, there’s still a great deal about epimorphic regeneration that remains to be solved. Today, I summarise insights from the following two articles:
On Regeneration after the Amputation of Abnormal Structures: I. Defective Amphibian Tails
D. R. Newth
https://doi.org/10.1242/dev.6.2.297
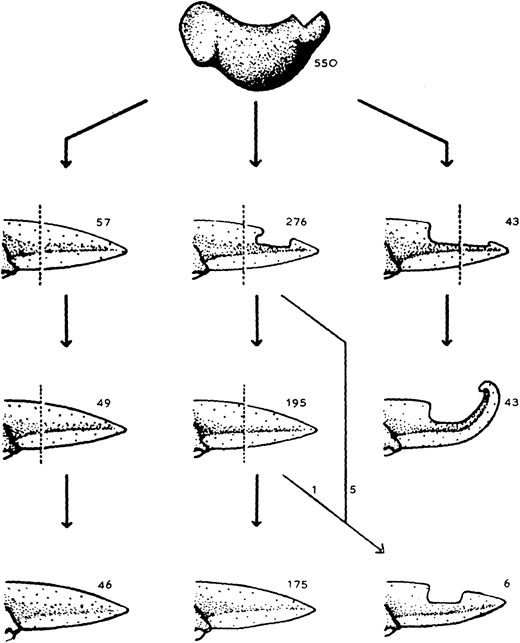
The almost-aptly-amphibian named David Richmond Newth was a Birmingham-born zoologist. After serving in India and Burma during the Second World War, he returned to the UK as a lecturer at Univesity College London. In his 1958 JEEM/Development publication (Newth, 1958), David sought to clarify some previous studies asking whether a regenerated amphibian tail would resemble the original: can animals restore defective appendages and regenerate structures that they didn’t have in the first place? Newth used both axolotl and the frog Rana temporaria as experimental systems, and operated on the embryos to induce the animals to develop an incomplete tail fin. In the frogs, he removed the dorsal half of the tail bud, whereas in axolotl he manipulated posterior neural folds to create ventral fin defects. Next, Newth amputated the tail at different positions along the anterior-posterior axis, cutting anterior to the defect or within the missing tissue. In the case of the Rana tadpoles, David repeated the amputation again (Fig. 1). As long as the amputation occurred anterior to the missing tail tissue, the amphibians could regenerate whole tails but dissection through the missing tissue did not generate complete tail outgrowth from the wound (Fig. 1). By showing that regeneration could form structures that were missing in the original appendage, this work indicates that embryonic development and regeneration might use different mechanisms of growth and patterning. However, the author is careful to point out that, in this case, the mechanism of regeneration in normal animals might not be the same as ‘abnormal’ ones; regeneration of ‘normal’ tissue could still follow developmental mechanisms. Second, this study demonstrates the importance of the position of amputation and indicates that the cells that form the blastema dictate which structures can be formed during regrowth.
It seems that the Axolotl is a species that has truly stood the test of time and is just as popular a model today as 500 years ago (Joven et al., 2019). However, technical scientific advances have advanced the toolbox available for asking complex research questions, including the generation of sophisticated axolotl genetic lines and reporters (e.g. Duerr et al., 2022). Although a wide range of frog species have been used in classical studies, present research largely concentrates on Xenopus spp. (Phipps et al., 2020). Whether regeneration employs the same development mechanisms to produce tissues during development remains unclear. Similar developmental signalling pathways (e.g. FGF, Shh) are involved in regenerating appendages; however, single-cell technologies are revealing that the progenitors deriving from the regeneration-specific organising centre – the blastema – have distinct molecular identities from those in the embryo and follow convergent, but different, trajectories (Čapek and Müller, 2019; Tsai et al., 2019). As we heard for wound healing yesterday, many recent investigations have focused on the roles of immune cells in amphibian tail regeneration, showing that myeloid cells and the factors they secrete are required for a regeneration-permissive environment (e.g. Tsai et al., 2019; Aztekin et al., 2020).
regeneration factors expressed on myeloid expression in macrophage-like cells is required for tail regeneration in Xenopus laevis tadpoles
Momoko Deguchi; Taro Fukazawa; Takeo Kubo
https://doi.org/10.1242/dev.200467
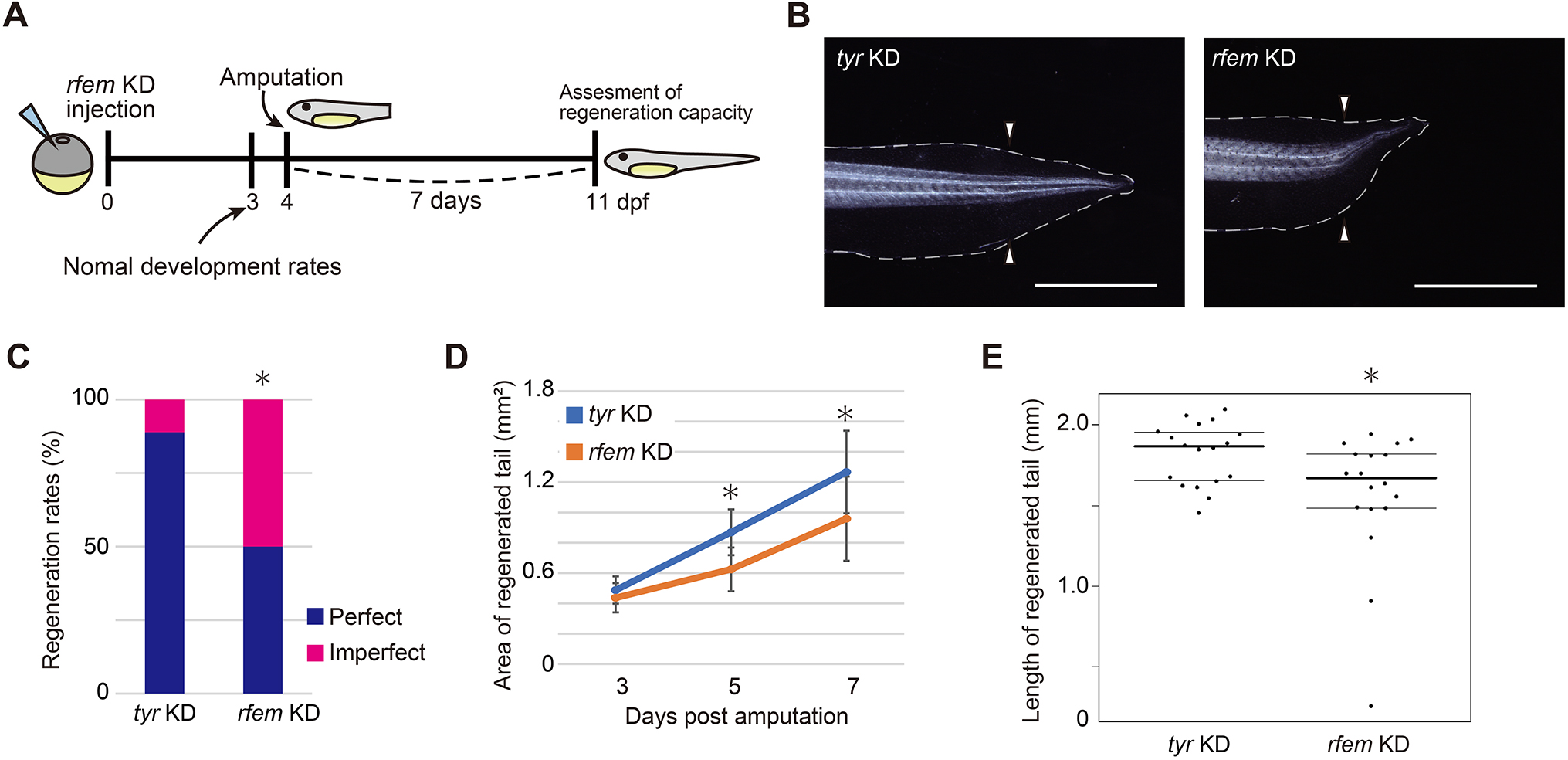
In their article published last month in Development, Deguchi and colleagues continue interrogating the intervention of the immune system in Xenopus laevis tail regeneration (Deguchi et al., 2023). Through a screen to identify cells that support progenitor proliferation, they find that regeneration factors expressed on myeloid (of which there are two paralogues in Xenopus, hereinafter collectively referred to as rfem) increases expression following tail amputation. It’s also required for regeneration because knockdown of rfem generates significantly shorter regenerated tails (Fig. 2). Using RNA sequencing of tail stumps and intact tails, the authors show that rfem-expressing cells cluster with leukocyte markers expressed by macrophages and dendritic cells. Deguchi and colleagues reduce the macrophage population, and thus rfem-expressing cells, by knocking down key monocyte-lineage gene csf1, which also caused tail regeneration abnormalities. Conversely, the addition of rfem-expressing macrophage-like cells in rfem knockdown animals rescues tail regeneration. Although the mechanism of Rfem activity is yet to be elucidated, this study builds upon previous research by identifying a factor through which immune cells might regulate epimorphic regeneration (Aztekin et al., 2020).
These two examples from the past and present both ask about the regenerative potential of the amphibian tail. Newth shows that regenerative programs can restore tissues that are effectively formed during development and aren’t possessed by the original structure and indirectly hints about various progenitor populations that might be involved. Deguchi and colleagues take this further to demonstrate that supporting cells, most likely macrophages, have an important role in regulating tail regeneration through the action of rfem-encoded proteins. However, this tale is far from over! Tomorrow, we indulge in a second helping of epimorphic regeneration, focusing on a dexterous but incredibly handy experimental technique: tissue grafts.
References
Can Aztekin, Tom W. Hiscock, Richard Butler, Francisco De Jesús Andino, Jacques Robert, John B. Gurdon, Jerome Jullien; The myeloid lineage is required for the emergence of a regeneration-permissive environment following Xenopus tail amputation. Development 1 February 2020; 147 (3): dev185496. doi: https://doi.org/10.1242/dev.185496
Daniel Čapek, Patrick Müller; Positional information and tissue scaling during development and regeneration. Development 15 December 2019; 146 (24): dev177709. doi: https://doi.org/10.1242/dev.177709
Ben D. Cox, Maximina H. Yun, Kenneth D. Poss; Can laboratory model systems instruct human limb regeneration? Development 15 October 2019; 146 (20): dev181016. doi: https://doi.org/10.1242/dev.181016
Momoko Deguchi, Taro Fukazawa, Takeo Kubo; regeneration factors expressed on myeloid expression in macrophage-like cells is required for tail regeneration in Xenopus laevis tadpoles. Development 1 August 2023; 150 (15): dev200467. doi: https://doi.org/10.1242/dev.200467
Timothy J. Duerr, Eun Kyung Jeon, Kaylee M. Wells, Antonio Villanueva, Ashley W. Seifert, Catherine D. McCusker, James R. Monaghan; A constitutively expressed fluorescent ubiquitination-based cell-cycle indicator (FUCCI) in axolotls for studying tissue regeneration. Development 15 March 2022; 149 (6): dev199637. doi: https://doi.org/10.1242/dev.199637
Alberto Joven, Ahmed Elewa, András Simon; Model systems for regeneration: salamanders. Development 15 July 2019; 146 (14): dev167700. doi: https://doi.org/10.1242/dev.167700
D. R. Newth; On Regeneration after the Amputation of Abnormal Structures: I. Defective Amphibian Tails. Development 1 June 1958; 6 (2): 297–307. doi: https://doi.org/10.1242/dev.6.2.297
Lauren S. Phipps, Lindsey Marshall, Karel Dorey, Enrique Amaya; Model systems for regeneration: Xenopus. Development 15 March 2020; 147 (6): dev180844. doi: https://doi.org/10.1242/dev.180844
Stephanie L. Tsai, Clara Baselga-Garriga, Douglas A. Melton; Blastemal progenitors modulate immune signaling during early limb regeneration. Development 1 January 2019; 146 (1): dev169128. doi: https://doi.org/10.1242/dev.169128


 (No Ratings Yet)
(No Ratings Yet)