A regeneration retrospective: go fish
Posted by Alex Eve, on 8 September 2023
This post is part of the regeneration retrospective series.
First, I’d be remiss not to acknowledge that most of my posts have been on vertebrate systems when many invertebrates are also studied for their regenerative capacity. One such example is Hydra (Vogg et al., 2019) but Hydra papers don’t seem to appear in JEEM/Development until the 1960s. While exploring the early issues of JEEM/Development, I found fish manuscripts were similarly rare, which is surprising because – today – zebrafish are a prominent model for development and regeneration (Marques et al., 2019). Indeed, there was also a notable absence of any studies of nervous system regeneration, maybe because such articles were submitted to more specialist neurobiology and neuroscience journals. In this final post of the series, we fill this missing link by discussing the highlights from the following articles:
Myotypic Respecification of Regenerated Nerve-fibres in Cichlid Fishes
H. L. Arora and R. W. Sperry
https://doi.org/10.1242/dev.5.3.256
Caltech researcher Roger Wolcott Sperry is most famous for his work on the ‘split brain hypothesis’ and the corpus callosum, for which he won the Nobel Prize for Medicine/Physiology in 1981 (Hubel, 1994; De León Reyes et al., 2020). Alongside his work in cats, Sperry published a few articles on cichlid fish. One such article was published in JEEM/Development in 1957 (Arora and Sperry, 1967). His colleague, R. W. Arora, seems to have left little trace but I think it’s likely he brought the fish to the whole operation. Their study continued previous work on the basis that humans failed to regenerate motor neurons because of aberrant guidance and muscle innervation following wounding or amputation, which doesn’t occur in other vertebrates. To learn more about peripheral nervous system (Murtazina and Adameyko, 2023) regeneration, Arora and Sperry focused on the mandible of Astronotus ocellatus, severing the left mandibular nerve of ten fish and causing partial paralysis had completely recovered by 16 days post-operation (Fig. 1, left). Next, they cut the individual branches of the mandibular nerve and crossed them so that the nerves lay next to muscles they wouldn’t normally innervate (Fig. 1, right). Overall, as with the complete nerve cuts, mandible function was recovered a couple of weeks later with the nerves innervating the adjacent muscle rather than their original target (I have simplified the results here, do see the manuscript for full details). Arora and Sperry even used an electric induction coil to prove innovation and show that stimulating the regenerated nerves would cause the contraction of the new muscle target. This work showed the plasticity of the peripheral nervous system to adapt to the rewiring of the motor neurons with new muscles. Furthermore, this study showed that the guidance cues that direct motor neuron migration and innovation during development might not be present during regeneration, signalling the importance of the microenvironment for pathfinding and differentiating the requirements for neuronal targeting vs. neuronal innervation.
.
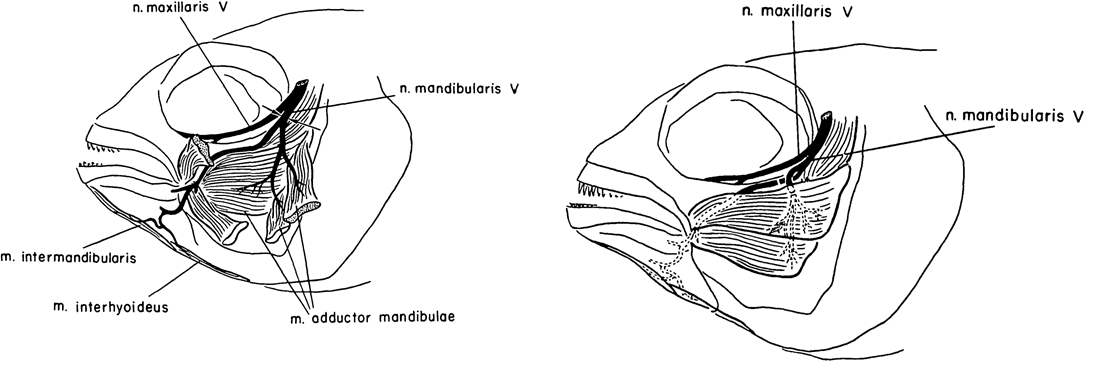
Teleost fish, including cichlids, are prominent in today’s research, although I think it’s fair to say that zebrafish are a dominant system. The explosion of zebrafish studies can largely be attributed to the Boston and Tübingen screens, culminating in the publication of a special issue of Development a little over 25 years ago (Nüsslein-Volhard 2012; Mullins et al., 2019). Since, the generation of hundreds (more?) of genetic lines, including fluorescent genetic reporters that capitalise on the zebrafish’s amenability to microscopy and imaging. As with cardiac regeneration, which we heard about earlier this week, the zebrafish (unlike most mammals, including humans) can regenerate its spinal cord (Becker and Becker, 2022).
Progenitor-derived glia are required for spinal cord regeneration in zebrafish
Lili Zhou, Anthony R. McAdow, Hunter Yamada, Brooke Burris, Dana Klatt Shaw, Kelsey Oonk, Kenneth D. Poss and Mayssa H. Mokalled
https://doi.org/10.1038/s41467-019-14263-2
In addition to the peripheral nervous system, zebrafish have a remarkable capacity for regenerating the central nervous system, including the spinal cord, following injury – a phenomenon that doesn’t occur in mammals due to glial cell-dependent scarring. Thus, understanding how to prevent scaring and/or induce regeneration has huge therapeutic potential for spinal cord injuries. In their recent Development paper, Zhou and colleagues use a suite of sophisticated genetic tools to ask how glial cells respond to injury in zebrafish (Zhou et al., 2023). The authors first generate ctgfa-Tracer zebrafish for lineage tracing ctgfa-expressing cells, such as bridging glia, which emerge following spinal cord injury. Using this line, the authors show that bridging glia, ventral ependymal progenitors and regenerating glial cells are derived from ctfga-expressing cells but they minimally contribute to neurons and oligodendrocytes. The authors next turn to ctfga regulation, revealing the gene-regulatory sequences that drive ctfga expression during regeneration. Finally, the authors specifically ablate ctgfa-expressing cells, and show that axon outgrowth and swimming behaviour are affected following spinal injury. Together, Zhou and colleagues determine that ctfga-expressing cells have a pro-regenerative role in spinal cord regeneration.
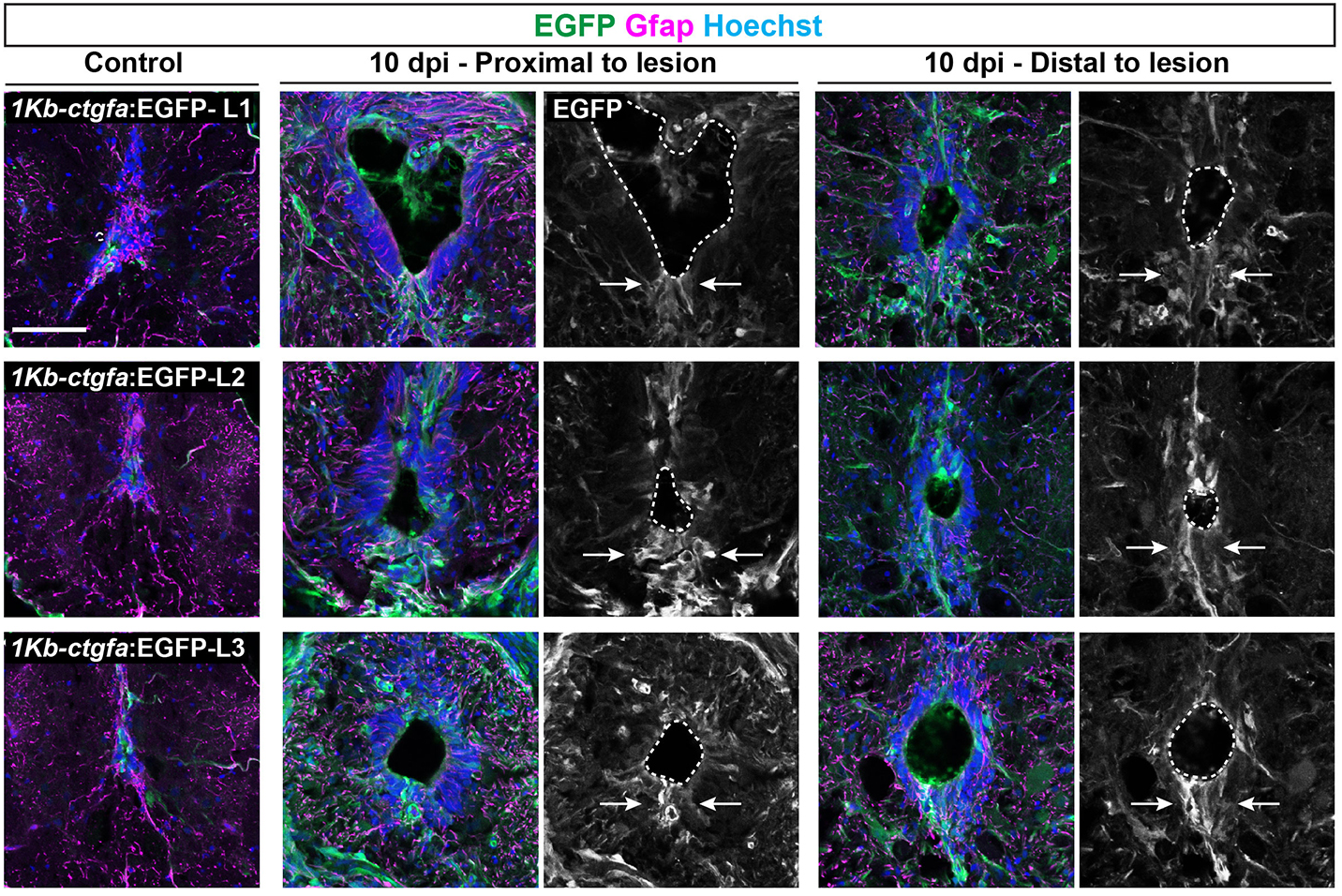
Both these papers address the ability of teleost fish to regenerate the nervous system and highlight the importance of the local microenvironment, as well as using animal behaviour (e.g. eating and swimming) as a read-out of phenotype. Arora and Sperry showed the regenerative plasticity of the peripheral nervous system, whereas Zhou and colleagues highlighted a particular cell population that supports and contributes to central nervous system regeneration.
I hope this little series has been entertaining – I’ve certainly enjoyed learning more about these early papers and the researchers who wrote them. Would you be interested in reading more ‘now and then’ or ‘past and present’ posts on other topics? Share your thoughts in the comments.
References
H. L. Arora, R. W. Sperry; Myotypic Respecification of Regenerated Nerve-fibres in Cichlid Fishes. Development 1 September 1957; 5 (3): 256–263. doi: https://doi.org/10.1242/dev.5.3.256
Thomas Becker, Catherina G. Becker; Regenerative neurogenesis: the integration of developmental, physiological and immune signals. Development 15 April 2022; 149 (8): dev199907. doi: https://doi.org/10.1242/dev.199907
D. Hubel; Roger W. Sperry (1913–1994). Nature 369, 1994; 186. https://doi.org/10.1038/369186a0
Noelia S. De León Reyes, Lorena Bragg-Gonzalo, Marta Nieto; Development and plasticity of the corpus callosum. Development 15 September 2020; 147 (18): dev189738. doi: https://doi.org/10.1242/dev.189738
Ines J. Marques, Eleonora Lupi, Nadia Mercader; Model systems for regeneration: zebrafish. Development 15 September 2019; 146 (18): dev167692. doi: https://doi.org/10.1242/dev.167692
Mary C. Mullins, Joaquín Navajas Acedo, Rashmi Priya, Lilianna Solnica-Krezel, Stephen W. Wilson; The zebrafish issue: 25 years on. Development 15 December 2021; 148 (24): dev200343. doi: https://doi.org/10.1242/dev.200343
Aliia Murtazina, Igor Adameyko; The peripheral nervous system. Development 1 May 2023; 150 (9): dev201164. doi: https://doi.org/10.1242/dev.201164
Christiane Nüsslein-Volhard; The zebrafish issue of Development. Development 15 November 2012; 139 (22): 4099–4103. doi: https://doi.org/10.1242/dev.085217
Stefano Sandrone. Roger W. Sperry (1913-1994). J Neurol. 2022 Sep;269(9):5194-5195. doi: 10.1007/s00415-022-11232-6. Epub 2022 Jul 22. PMID: 35867150; PMCID: PMC9363358.
Matthias C. Vogg, Brigitte Galliot, Charisios D. Tsiairis; Model systems for regeneration: Hydra. Development 1 November 2019; 146 (21): dev177212. doi: https://doi.org/10.1242/dev.177212
Lili Zhou, Anthony R. McAdow, Hunter Yamada, Brooke Burris, Dana Klatt Shaw, Kelsey Oonk, Kenneth D. Poss, Mayssa H. Mokalled; Progenitor-derived glia are required for spinal cord regeneration in zebrafish. Development 15 May 2023; 150 (10): dev201162. doi: https://doi.org/10.1242/dev.201162


 (No Ratings Yet)
(No Ratings Yet)