Aging stem cells
Posted by Erin M Campbell, on 12 October 2011
There are so many factors for a stem cell to consider when deciding cell fates. A recent paper from Development discusses how the age of a stem cell can affect its future.
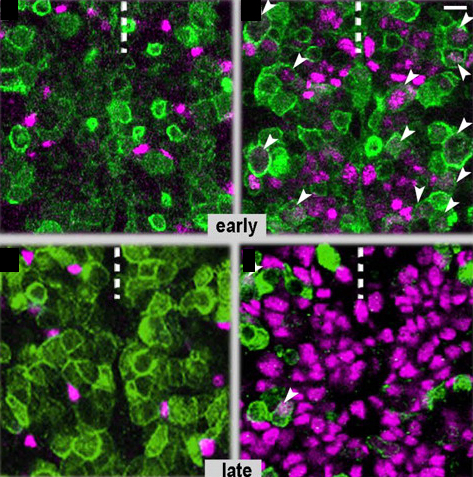
Neurons and glial cells are two major cell types in the nervous system, and both come from the many divisions of neural stem cells (NSCs). The amazing plastic characteristics of NSCs drive a lot of excitement over their future use in regenerative medicine, but the complex gene network in vertebrates makes understanding NSC plasticity difficult. Flici and colleagues recently published a paper on NSC cell fate decision-making in the simple CNS of fruit flies. The transcription factor Gcm was already known to drive glial fate in NSCs. Flici and colleagues found that overexpression of Gcm in NSCs forced a complete conversion to glial cells. In addition, NSCs plasticity is affected by age—as NSCs get older, their ability to drive glial cell fates decreases. After NCSs fell into a quiescent state at old age, Gcm overexpression was no longer able to force glial cell conversion, suggesting that temporal cues, not mitotic potential, drive NSC plasticity. Finally, Flici and colleagues found that the Gcm-glial cell fate pathway leads to low levels of H3K9ac, which is similar to the low levels of histone acetylation seen in vertebrate glial cells. In the images above, fly embryos are labeled to show neurons (green) and glial cells (purple). Control embryos (left) have few glial cells, while embryos with Gcm overexpression (right) have many glial cells. The longer the Gcm overexpression, the more glial cells develop at the expense of neurons (top is early, bottom is late). Arrowheads show cells with markers for both glial cells and neurons, an intermediate stage in the conversion towards glial fate.
For a more general description of this image, see my imaging blog within EuroStemCell, the European stem cell portal.
Flici, H., Erkosar, B., Komonyi, O., Karatas, O., Laneve, P., & Giangrande, A. (2011). Gcm/Glide-dependent conversion into glia depends on neural stem cell age, but not on division, triggering a chromatin signature that is conserved in vertebrate glia Development, 138 (19), 4167-4178 DOI: 10.1242/dev.070391


 (2 votes)
(2 votes)