Behind the paper: How veiled chameleons (Chamaeleo calyptratus) make their left and right sides.
Posted by Natasha Shylo, on 15 May 2023
Dr. Natalia (Natasha) Shylo, Dr. Paul Trainor and colleagues at Stowers Institute for Medical Research in Kansas City, Missouri, USA recently published an article in Frontiers in Cell and Developmental Biology, titled “Morphological changes and two Nodal paralogs drive left-right asymmetry in the squamate veiled chameleon (C. Calyptratus).” Here is a behind the scenes look into using veiled chameleons as a research organism.
How did you get started on this project?
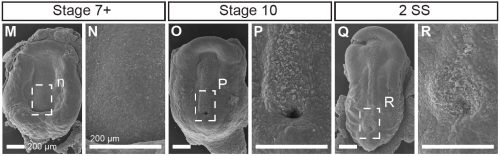
This project began like a lot of very cool studies do, completely accidentally. We were wrapping up a project describing neural crest cell patterning in veiled chameleons started by a former PhD student Dr. Raul Diaz, and Dr. Natasha Shylo created a staging series of veiled chameleon embryo development to go with the study (Diaz et al., 2019). Based on her prior work in left-right patterning, Dr. Shylo then recognized the potential of veiled chameleons to understand how left-right patterning occurs in non-avian reptiles. When we started this work in 2019, nothing was known about left-right patterning in non-avian reptiles. And it was going to be easy (famous last words), since we already had some of the key reagents – Shh and Fgf8 RNA in situ probes from our previous work in chameleon limb patterning (Diaz and Trainor, 2015), and a Nodal probe for a gastrulation study (Stower et al., 2015). Shh was the first molecular marker ever published with asymmetric molecular expression in chicken, so it was a logical starting point. Also given the presence of cilia and their known roles in left-right organizer function, we also searched for cilia using SEM. But Shh was not asymmetrically expressed in veiled chameleon embryos, and we found nothing that resembled motile cilia in the blastopore region of the embryo.
What was known about left-right patterning in non-avian reptiles before your work?
Left-right patterning in non-avian reptiles was a black box when we started this project. However, we were not the only ones curious to tackle this question. As we were trying to figure out the steps of the Nodal signaling cascade and what happens in an organism that doesn’t use cilia in its left-right organizer, Dr. Hiroshi Hamada’s group published a paper revealing that turtles and geckos also do not use motile cilia to establish left-right patterning (Kajikawa et al., 2020). We were able to build on those observations and realized we were looking at the “wrong” Nodal gene. Nodal1, as we now refer to it, was not supposed to exist in chameleons. It is the only Nodal paralog present in mammalian genomes, but has been lost from the genomes of chickens, geckos, and turtles, which retained Nodal2 instead. It turned out that the veiled chameleon genome contains two Nodal genes – both retained from a duplication event in jawed vertebrates.
What made you choose the veiled chameleon as your model organism?
Although we have a good understanding of early developmental events in many vertebrate model organisms, few studies if any involved the reptile clade. This is because at the time lizards and snakes lay their eggs, their embryos are already at limb bud stages of development, which is far too late to study left-right patterning, gastrulation and neurulation. We became interested in veiled chameleons because their embryos are pre-gastrula stage at the time of oviposition and because of their sexually dimorphic casque, which we hypothesized was neural crest cell derived. Fortunately, veiled chameleons are a popular pet with established husbandry. They breed year-round and lay large clutches of eggs. Dr. Shylo came to the lab with expertise in left-right patterning, and veiled chameleons proved to be a perfect research organism to study the mechanisms establishing left-right asymmetry in non-avian reptiles.
How is it like working with veiled chameleons?
One must be patient when working with veiled chameleons because their development is really slow. It can take up to 70 days to accomplish gastrulation, and a week to establish left-right patterning, compared to about 6 hours in mice, which means veiled chameleons allow for a much finer temporal resolution of key developmental processes. We stagger chameleon mating, and consequently always have an abundance of embryos at various stages of development throughout the year. Veiled chameleon eggs are soft shelled, which means we can’t manipulate the embryos in ovo like chicken embryos, but they are amendable to ex ovo culture, lineage tracing, time lapse imaging and most molecular techniques.
Can you summarise your key findings?
We used veiled chameleons to study the evolution of developmental mechanisms and obtain a deeper understanding of how left-right patterning is established in non-avian reptiles. We found that like chickens, veiled chameleons do not use motile cilia in their left-right organizer to establish left-right asymmetry. Instead, through live imaging, we observed asymmetric morphological changes in the embryo, which precede, and likely initiate molecular asymmetry. We further discovered that veiled chameleons have retained two Nodal genes – Nodal1 and Nodal2 – from a duplication event in jawed vertebrates. In comparison, mammals have only Nodal1, whereas avians have retained Nodal2. This work has laid the foundation for our future studies, aimed at understanding in greater detail the mechanisms and processes that establish left-right asymmetry in diverse groups of amniotes.
Were you surprised to find that the veiled chameleons do not have motile cilia in their left-right organizer?
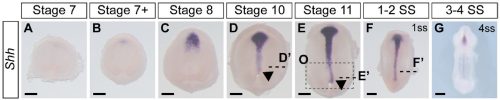
Not entirely. Going into this project, the outcome was always going to be binary – they will have motile cilia or not. It has been known for a long time that avian embryos do not have motile cilia in their left-right organizer, so it was reasonable to expect that all reptiles might be the same. What was surprising was the pattern of Shh expression, because Shh is expressed asymmetrically around the node in chickens and pigs (pigs also lack motile cilia in their left-right organizer). In veiled chameleon embryos, Shh is expressed symmetrically in the floor plate, but major morphological changes in the embryo push the Shh expressing tissues to the left. In another surprise and again distinct from chickens and pigs, veiled chameleons don’t use a primitive streak for gastrulation, and appear to lack a morphological node structure. We presume that the blastopore slit in chameleons carries out the function of the left-right organizer, but this remains to be determined.
Did you have any particular result or eureka moment that has stuck with you?
We knew that imaging development live would be key to our understanding of left-right patterning in chameleons, and we collected multiple movies at various stages of development, but no matter how we squinted, tilted our heads, or what type of analysis we used, we could not find consistent robust asymmetric cellular movements in the embryos we imaged. However, in a fortuitous meeting with collaborator and co-author Dr. Sarah E. Smith, viewing the movies in the transverse optical plane revealed an unmistakable asymmetric tilt that occurred reproducibly in all of our movies, which pushes the Shh expression tissue to the left at the same precise stage of development! We still think that the gross morphological rearrangement that we reported is driven by finer cellular movements, and we will continue our work with chameleons to figure out where, when, and how it all starts.
And the flipside: were there any moments of frustration or despair?
As mentioned earlier, Shh was the first molecular marker that we characterized, and it appeared perfectly symmetrical at every stage that we initially examined. This observation was consistent with a report in turtles and geckos from Dr. Hiroshi Hamada’s lab. Apparently, we had a model system in which the left-right organizer lacked motile cilia, and Shh was symmetrically expressed, and this contradicted everything we knew from mice (motile cilia) and chickens (asymmetric Shh). However, it made some sense, since chameleons don’t have a conventional node that rotates. As we started to prepare the manuscript, Dr. Shylo realized a broader time course of Shh expression was required, and there it was, unmistakable asymmetrically displaced Shh expressing tissue! Only these developmental stages were much earlier than when asymmetric Nodal expression is observed! Thus, an initial moment of frustration turned into weeks of excitement. We had simply been looking too late in development. These data revealed that morphological changes occur in a veiled chameleon embryo well before we can detect asymmetric Nodal expression, opening up new models and mechanisms to explore.
Natasha – What brought to you join Paul’s lab? And what is next for you after this paper?
My Ph.D. focused on the ciliary gene Tmem107 in a mouse mutant which exhibited left-right patterning defects (Shylo et al., 2020). Work with a collaborator revealed that these mice also had a neural crest defect, which made me curious about roles for cilia in neural crest cell specification and migration (Cela et al., 2018). So I came to Paul’s lab with a plan to study neural crest cells in mice, but I will continue to study the evolution of developmental mechanisms, particularly with respect to left-right patterning and gastrulation.
I plan to seek out a faculty position in the next year or two, and establish a laboratory to study gastrulation, left-right patterning, and other early developmental processes, using veiled chameleons as a model for early amniotic development. Although my focus has changed dramatically through a series of serendipitous events, there is no other place I could have done this work as efficiently as in Paul’s lab at Stowers Institute. To my knowledge, we maintain the largest colony of veiled chameleons used specifically for research. We have recently sequenced the veiled chameleon genome and once the annotation is finalized, we will have all the genetic, molecular and cellular tools we need to functionally study the evolution of developmental mechanisms with an emphasis on early development in these reptiles. My message to other postdocs based on my experience is to be brave, and if an amazing project summons your attention, it is OK to completely switch directions and your scientific focus. Go for it.
Paul – Where will this story take the lab?
Veiled chameleons exhibit a number of interesting morphological features including a cranial casque, forelimb and hindlimb clefting, and wrists and ankles that function like balls and sockets instead of hinges. Chameleons also have a projectile tongue, a prehensile tail, and they can color change. All of these features make chameleons very well suited for arboreal environments. With annotation of our newly sequenced genome nearly complete, we will soon be able to tackle functional genetic questions in ecological-evolutionary-developmental biology using veiled chameleons in concert with traditional model organisms.
References
CELA, P., HAMPL, M., SHYLO, N. A., CHRISTOPHER, K. J., KAVKOVA, M., LANDOVA, M., ZIKMUND, T., WEATHERBEE, S. D., KAISER, J. & BUCHTOVA, M. 2018. Ciliopathy Protein Tmem107 Plays Multiple Roles in Craniofacial Development. J Dent Res, 97, 108-117.
DIAZ, R. E., JR., SHYLO, N. A., ROELLIG, D., BRONNER, M. & TRAINOR, P. A. 2019. Filling in the phylogenetic gaps: Induction, migration, and differentiation of neural crest cells in a squamate reptile, the veiled chameleon (Chamaeleo calyptratus). Dev Dyn, 248, 709-727.
DIAZ, R. E., JR. & TRAINOR, P. A. 2015. Hand/foot splitting and the ‘re-evolution’ of mesopodial skeletal elements during the evolution and radiation of chameleons. BMC Evol Biol, 15, 184.
KAJIKAWA, E., HORO, U., IDE, T., MIZUNO, K., MINEGISHI, K., HARA, Y., IKAWA, Y., NISHIMURA, H., UCHIKAWA, M., KIYONARI, H., KURAKU, S. & HAMADA, H. 2020. Nodal paralogues underlie distinct mechanisms for visceral left-right asymmetry in reptiles and mammals. Nat Ecol Evol, 4, 261-269.
SHYLO, N. A., EMMANOUIL, E., RAMRATTAN, D. & WEATHERBEE, S. D. 2020. Loss of ciliary transition zone protein TMEM107 leads to heterotaxy in mice. Dev Biol, 460, 187-199.
SHYLO, N. A., SMITH, S. E., PRICE, A. J., GUO, F., MCCLAIN, M. & TRAINOR, P. A. 2023. Morphological changes and two Nodal paralogs drive left-right asymmetry in the squamate veiled chameleon (C. calyptratus). Frontiers in Cell and Developmental Biology, 11.
STOWER, M. J., DIAZ, R. E., FERNANDEZ, L. C., CROTHER, M. W., CROTHER, B., MARCO, A., TRAINOR, P. A., SRINIVAS, S. & BERTOCCHINI, F. 2015. Bi-modal strategy of gastrulation in reptiles. Dev Dyn, 244, 1144-1157.


 (No Ratings Yet)
(No Ratings Yet)