Behind the Story: Immunity, Involution and Indefatigable Women
Posted by ChrisJWatson, on 19 July 2022
Christine J Watson, Department of Pathology, University of Cambridge, Tennis Court Road, Cambridge CB2 1QP
Email: cjw53@cam.ac.uk

We are excited that our study on immune cells, post-lactational regression of the mammary gland, and tumour growth has been published in a Special Issue of Development on The Immune System in Development and Regeneration. This paper is the culmination of many years of work by a fantastic team of women. For over two decades, my laboratory has been interested in the mechanisms of cell death that are utilised after weaning to remove the milk-producing alveolar cells in the lactating mammary gland as they are no longer required. The mouse mammary gland is a fantastic experimental tool with which to investigate cell death under physiological conditions as the lobuloalveolar structures that make milk during lactation arise de novo, with each and every pregnancy, and are subsequently removed when lactation ceases. The use of milk protein gene promoters to drive expression of Cre recombinase has allowed us to conditionally delete any gene of interest specifically in the alveolar cells and so we can investigate the role of specific genes in lactation and cell death during involution without perturbing normal mouse physiology.
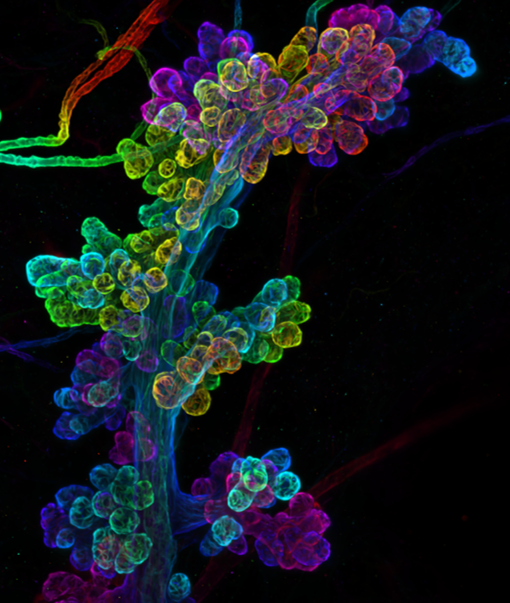
We showed way back in 1998, using the beta-lactoglobulin promoter to drive Cre expression, that the transcription factor Stat3 was essential for initiating cell death during involution. This was a wonderful collaboration with Rachel Chapman and the late Alan Clarke when our laboratories were based in Edinburgh. We continued our work on the mechanism of Stat3-mediated cell death after my laboratory moved to Cambridge and we were joined by a talented postdoc, Sara Pensa, who had done her PhD with a long-standing collaborator Valeria Poli from the University of Turin in Italy. Valeria’s lab has considerable expertise in Stat3 signalling, cancer and immunology. Among other things, Sara was interested in looking at the effect of age on mammary tumour growth and she initiated work to investigate this in mice. We were joined by a veterinary surgeon and histopathologist, Kate Hughes, who elected to do her PhD in my laboratory and another talented postdoc Bethan Lloyd-Lewis who brought considerable expertise in mammary gland, gained in Trevor Dale’s laboratory in Cardiff. Bethan had an interest in developing lineage tracing and imaging technologies to investigate mammary stem and progenitor cells. Combining these interests and technologies allowed us to investigate multiple aspects of tumour growth utilising a cell culture model of human epidermal growth factor receptor 2 (HER2) overexpression, used in Valeria’s laboratory, and called TUBO cells. HER2 is overexpressed in a subtype of breast cancer that affects about 1 in 5 women with breast cancer usually as a result of the gene encoding HER2 being present in multiple copies. Although this is a more aggressive type of breast cancer, the use of a humanised monoclonal antibody that targets HER2, called Trastuzumab, in combination with other therapies, has proved beneficial.
We decided to use the TUBO cell line as it is a reliable and predicable model of mammary cancer development. We wanted to investigate how the involution process, with its associated extensive cell death and tissue remodelling, would affect the growth of tumours arising from implanted TUBO cells. It is established that the involution process is associated with a transient increase in the risk of developing breast cancer in women, called post-partum breast cancer. However, a full-term pregnancy before the age of 30 reduces the lifetime risk of breast cancer while childbirth after the age of 35 does not provide any protection. It is not well understood how undergoing a full lobuloalveolar development programme during pregnancy can protect from cancer at a young age, why this is abrogated in older mothers, and why the involution process can be pro-tumourigenic. We wished to gain some insights into the molecular and cellular events behind these observations. An interesting aspect of mammary gland involution is that there is an array of immune cell types present in the gland and a dramatic influx of immune cells around day 3 of involution when extensive phagocytosis of dead alveolar cells, milk fat and cellular debris is required along with remodelling of the extracellular matrix and redifferentiation of the white adipocytes in the mammary fat pad. It is remarkable that these processes do not cause overt inflammation. We realised that our team was missing an expert immunologist and we were fortunate to recruit a new postdoc with such expertise. Jessie Hitchcock had just completed her PhD at the University of Birmingham on immunity to infection, focussing on systemic inflammation, and she was keen to move into the cancer field. So, with Jessie on board, we were now well placed to carry out an extensive study on the growth of tumour cells transplanted into involuting mammary glands at various stages.
We were able to analyse immune cells and tumour growth in the mouse mammary gland using a variety of techniques combining our various expertise: histology, deep 3D imaging, flow cytometry, tumour cell implantation and tumour growth measurement. Jessie showed, surprisingly, that leukocytes (marked by CD45 expression) are present not only in the tissue stroma but that a subset intercalate between the myoepithelial and luminal epithelial cells in the ductal epithelial bilayer in virgin mammary gland while during lactation/early involution, these leukocytes co-localise with myoepithelial cells and have a very different shape similar to the star-like morphology of dendritic cells. As observed by 3D immunofluorescent imaging, the density of CD45+ cells in both the epithelium and stroma is greatest at 3 days after forced weaning, and decreases quite dramatically by day 6 of involution, with the leukocytes associating less with the contracting myoepithelium at this stage. Jessie also carried out an extensive analysis of immune cell types present in the mammary gland by flow cytometry at various stages of involution and also in pregnant and non-pregnant mice. Our tumour experiments focussed on injecting TUBO cells into mammary glands of both young and old syngeneic mice at different stages of involution followed by monitoring tumour growth. Syngeneic mice were essential to allow us to investigate whether the fluxes in immune cell types, that we had observed by flow cytometry, had an influence on initial tumour growth.
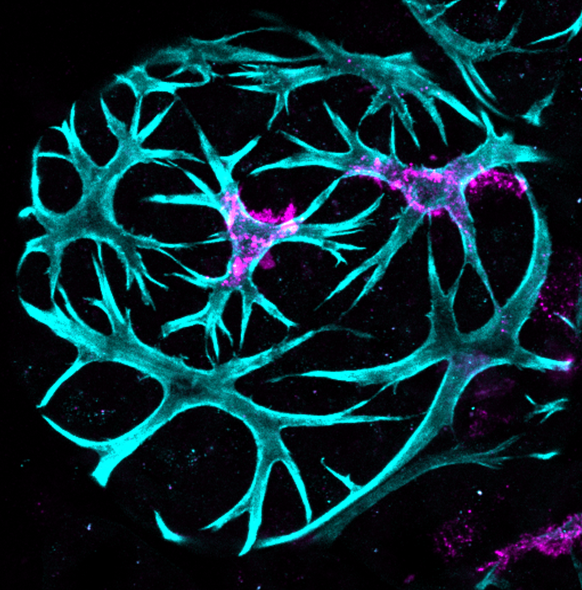
These were challenging experiments, but we generated some interesting data. Firstly, and surprisingly, we found that the environment in the mammary gland at day 3 involution promoted faster tumour growth compared to nulliparous mice while the environment at day 6 involution suppressed tumour growth considerably compared to day 3 involution and tumours were even slower growing than in nulliparous mice. We were able to correlate these changes in tumour growth rate with the immune cell types present in the gland at these times, and particularly with distinctly elevated CD11b-expressing macrophage populations, that may express inflammatory genes, at day 6 involution compared to day 3 involution. We also found that tumours tend to grow faster at day 3 involution in aged mice (10 months old, equivalent to 38 years of age in women) compared to young mice. Despite differences in growth rate, the immune environment, and the age of mouse, all tumours appeared morphologically similar when assessed both histologically and by 3D imaging of optically cleared tumour tissue.
This work has provided a basis for preclinical studies in women’s breast cancers and for characterisation of weaning-induced breast involution in young women. Furthermore, our study highlights the merits of multidisciplinary work and collaboration between a team of talented and enthusiastic scientists.


 (2 votes)
(2 votes)