BSDB Gurdon Studentship Report – Sonia Paoli
Posted by Sonia Paoli, on 6 January 2023
Exploring how germ cells are metabolically supported in Drosophila testes
During the summer I had the privilege to work in Dr. Amoyel’s lab at UCL, to study the mechanisms providing metabolic support to germ cells in Drosophila testes. As my first lab project, it was thrilling to realize how much I enjoy academic research and feel invested in shining light on the unknown.
The lab seeks to understand how stem cell interact with their environment and with each other, to influence their fate and maintain tissue homeostasis. In fact, stem cells can either undergo self-renewal or differentiation, to fulfil their functions in tissue maintenance and regeneration. The balance between these two fates is determined by a micro-environment called a niche, which delivers signals promoting proliferation to the adjacent stem cells, while cells that are displaced from the niche differentiate. In particular, the Drosophila testis niche, called the hub, supports two stem cell lineages, namely germline stem cells (GSCs), which differentiate into germ cells, and somatic stem cells (CySCs), differentiating into post-mitotic cyst cells. When differentiating, two somatic cyst cells (CCs) envelop one germ cell (GC), forming a complex called a cyst (See Fig. 1). The enclosed GC then undergoes several mitotic events, increasing its number within the cyst from 1 to 16 cells (See Fig. 2).
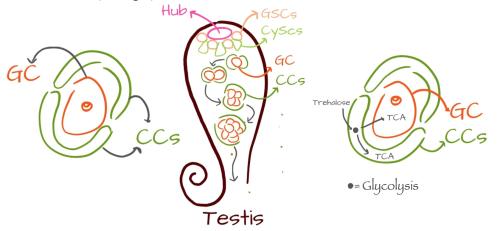
Fig. 2 Drosophila testis, containing the hub and two cell lineages. A differentiated germ cell undergoes mitotic events within a cyst.
Fig. 3 In a cyst, our theory is that CCs provide glycolysis products to support TCA in both cell lineages.
Since the enclosed GCs are sealed off from the environment by the two somatic CCs, the way they receive the metabolites necessary for the glycolytic and TCA pathways to survive was still unknown. In carbon metabolism, sugars – Trehalose molecules in flies – are broken down through a process called glycolysis, which produces pyruvate and ATP. Pyruvate is then either converted to lactate, or transported into mitochondria to fuel the TCA cycle, which provides most of a cell’s energetic needs. Hence, my project’s objective was to understand whether the two stem cell lineages interact metabolically, exploring the hypothesis that CCs provide glycolysis products to support germ cell metabolism (See Fig. 3). This shuttling of metabolites is already known to happen in the brain, where glia conduct glycolysis and provide lactate to neurons to feed their mitochondria. To test this theory in Drosophila testes, we knocked out glycolytic and TCA enzymes to downregulate the respective metabolic processes. A prediction of our hypothesis is that different metabolic pathways should be required in different cell types. In particular, knocking down glycolysis only in CCs should affect GCs, while knocking it down in GCs should have no effect, while both cell types should rely on TCA cycle enzymes.
During the first week, I learnt about Drosophila handling and husbandry: the basics about fly development and genetics, how to set up crosses, and how to identify markers to select the correct flies for analysis. I was also trained in immunostaining, to identify cell types in the tissue and whether their distribution changed as a result of my genetic manipulations. With plenty of practice and patience, I was soon comfortable enough with the staining procedure to start building my own experiments, and that’s how slowly the puzzle finally started coming together and I found myself in charge of boxes of crosses, and creating my own routine in order to keep crosses and experiments going.
This project was also an opportunity for me to discover and apply the Gal4/UAS-RNAi system, one of the main techniques used to conduct large-scale gene disruption in flies. Gal4 encodes a transcription factor which specifically binds to an enhancer called UAS, and activates expression of target genes downstream of the UAS sequence. Spatial control of expression is achieved by placing Gal4 expression under the control of tissue-specific promoters. When RNA interference (RNAi) sequences are placed downstream of UAS, their expression inhibits the expression of targeted proteins, disrupting gene expression. Therefore, when crossing a female fly carrying a Gal4 transgene with a male carrying a UAS-RNAi for a specific metabolic enzyme, the enzyme will be knocked down in the progeny carrying both transgenes.
To test these hypotheses, I used specific Gal4 drivers, including Traffic jam (Tj-Gal4), which targets CCs, Nanos-Gal4, which is only expressed in GCs, and Tubulin (Tub)-Gal4, which is expressed in all cells. These metabolic genes should all be essential for viability during development, so knocking them down in all cells using the UAS-RNAi system should result in lethality and a failure to obtain offspring carrying both the Gal4 and UAS-RNAi transgene.
My results showed that knocking down all glycolytic and TCA enzymes with Tub-Gal4 led to lethality, except for knockdown of Trehalose transporters (Tret), which did not affect viability. This might indicate either that the many versions of Tret proteins overlap each other in their functions, or that there are other pathways that might lead to the same downstream outcome without the use of Tret enzymes. Together with Holly Jefferson, we went on to show that knocking down glycolysis genes in CCs led to decreased GC survival, while knockdown in GCs had no effect. These results altogether support the hypothesis that cyst cells produce metabolites through glycolysis to support germ cell metabolism.
The sense of responsibility I felt, and the way other lab colleagues, with many more years of experience than me, relied on me to complete the necessary experiments, was in a sense enlightening for me to understand dynamics in a laboratory and in a work environment. A dynamic in which colleagues confide in each other’s’ abilities and strengths, in which every question is a good question, and in which a wrongly dissected testis or a failed experiment is an opportunity to learn and fully comprehend mechanisms without blindly following protocols. This wholesome experience has shown me how working in research, contributing to the scientific community, and entering the world of scientific discovery, is a life full of suspense and serendipity, struggle and competition, failure and accomplishment. It is a selfless life to which my future career aspirations lean fully, allowing me to explore the mechanisms through which life is possible.
Sonia Paoli – UCL, BSc Biomedical Sciences, Cells and Molecules
Supervisors: Marc Amoyel, Diego Sainz de la Maza, Holly Jefferson


 (4 votes)
(4 votes)
Very proud of you Sonia.
Great work and writing. You will have to explain a lot to a neonatologist.
Dr. Desai
Great work, Sonia. Thanks to all your mentors who guided you through this research work. Dr. Anand