The people behind the papers – Daniel Osório, Elaine Chan, Joana Saramago and Ana Carvalho
Posted by the Node Interviews, on 13 January 2020
This interview, the 73rd in our series, was recently published in Development.
Animal cytokinesis is driven by an actomyosin ring that assembles at the cell equator and constricts to physically separate the two daughters. Although myosin is known to be essential for cytokinesis in multiple systems, whether this requirement reflects its motor or actin crosslinking activities has recently been a matter of contention. A new paper in Development now addresses this problem using the first divisions of the Caenorhabditis elegans embryo as a model. We caught up with the paper’s three first authors Daniel Osório, Elaine Chan and Joana Saramago, and their supervisor Ana Carvalho, Principal Investigator at the University of Porto’s i3S consortium, to find out more about the story.

Ana, can you give us your scientific biography?
AC I graduated in biochemistry from the Faculty of Sciences of the University of Porto and did my doctoral studies on mitosis with Professor Bill Earnshaw at the Wellcome Trust Centre for Cell Biology in Edinburgh, UK. Then I moved to San Diego, USA, to do my postdoctoral work on cytokinesis in Professor Karen Oegema’s group at the Ludwig Institute for Cancer Research. In 2012, I moved to IBMC, the Institute for Molecular and Cell Biology in Porto, where I launched my independent research group. IBMC is currently part of the i3S, Institute for Research and Innovation in Health, a large consortium of three research institutes headed by the University of Porto.
Daniel, Elaine and Joana: how did you come to work in the Carvalho lab, and what drives your research today?
DO When I finished my PhD with Edgar Gomes in Paris working on the cell biology of nuclear positioning mediated by actin-binding proteins, I was looking to return to Portugal and my soon-to-be wife, having been away for almost 7 years. I found that the Carvalho lab had been recently established and had an opening in a C. elegans project to work with actomyosin and cytokinesis. In 2009 I had been one of the lucky ones selected for the physiology course of the Marine Biological Laboratory in Woods Hole, and during that course I got my first contact with C. elegans by interacting with the Oegema-Desai labs where Ana did her post-doc. Although we did not meet at the time I was very impressed by the model and its possibilities, so I guess the universe aligned later on and I was selected by Ana to take on this ambitious project.
EC In 2012, I got married to my Portuguese husband. After marriage, we decided to move from San Diego to Portugal, but at that time finding a research job in Portugal was not easy because of the economic crisis. But I did not give up my research dream. I contacted Ana because I really liked her research projects studying cell division in C. elegans, which was similar to my PhD background on bacterial cell division. After meeting her, we realised that we had both worked in the same university in San Diego before moving to Portugal, just in different buildings. We never had a chance to meet on the UCSD campus but we got our chance in Portugal! Ana and I have been working on actomyosin projects together for almost 7 years.
JS In 2013, I started my Master’s thesis and I knew that I wanted to do cell biology, and I started to search for an interesting lab. I visited a few labs, but when I saw a beautiful C. elegans embryo undergoing cytokinesis on Ana’s computer I knew that I had found what I was looking for. I also loved the idea of doing live microscopy, which was a dream of mine since I was a little girl: to see what our eyes can’t see. I liked it so much that I stayed to do my PhD after my Master’s.
Why has myosin’s precise role in cytokinesis been controversial?
DO Well, first and foremost because if you deplete or inactivate myosin, cytokinesis fails in most situations and systems. This only allows for the conclusion that myosin is required for the process, not that myosin motor activity is required. Second, I think it has been controversial because we tend to take the concept of conservation of processes too far: we want to assume that cytokinesis in a small yeast cell works exactly like it does in adhered cells or in a C. elegans embryo where divisions are so fast. Although the players and mechanisms are clearly conserved and a lot has been learned from studies in all systems from yeast to mammalian cells, we should accept that there may be system-specific variations. These should not be looked at as controversies, but may in fact reflect differences between the systems. Finally, the lack of good structural and quantitative information on the actomyosin organisation of the contractile ring in animal cells has limited the interpretation of phenotypes. Recent work using both electron and super-resolution microscopy are improving knowledge in the field.
Can you give us the key results of the paper in a paragraph?
DO, EC, JS & AC The motor activity of non-muscle myosin II is essential for cytokinesis and contractile ring contractility in C. elegans embryos, and the motor activity of muscle myosin II is required for contraction but dispensable for actin organisation in the body muscle of C. elegans adults.
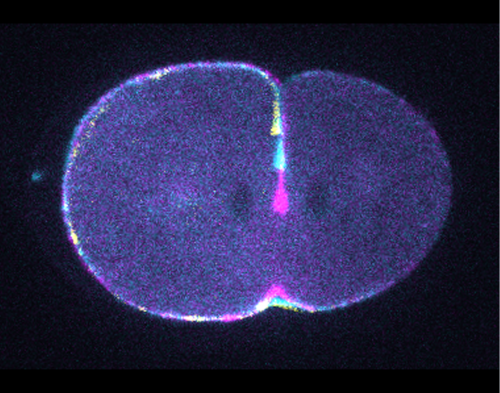
Why do you think myosin motor mutants have a slower rate of contractile ring constriction?
DO Although several mechanisms may be involved, our work suggests that actin filament sliding through myosin motor activity is indeed important for constriction of the contractile ring. This is in agreement with previous proposals and with the organisation of the actomyosin network observed in recent electron microscopy and super-resolution studies. Therefore, mutants with reduced or absent motor activity have a reduced or absent ability to slide actin filaments, thus presenting a reduced rate of ring constriction.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
DO I would not say eureka moments but there were some particular events that definitely contributed to the advance of the story. One was testing the effects of myosin mutations in the muscle. Although muscle and non-muscle myosins have biochemical differences and the muscle has a different organisation when compared to what we know about the contractile ring, we wanted an in vivo strategy that could help validate in vitro results as a complementary approach. Additionally, since we were not able to use animals expressing just the motor dead mutants (as they were sterile and did not produce embryos), the use of a wild-type copy from another location in the genome that could specifically be depleted by RNAi was a seemingly simple but very important step. Finally, getting the in vitro myosin motility assay to work was important. Although it has been established for many years, it is quite a challenging and meticulous process requiring several proteins to be expressed and purified in a functional form. This was quite time-consuming and not straightforward – in a sense I consider it a eureka moment for the lab because we started from scratch.
AC Being able to generate C. elegans in which the only source of UNC-54, the major muscle myosin, is UNC-54(R710C) and observing them moving normally was an important moment. Previous work has considered the equivalent mutation in non-muscle myosin II from mammalian cells to be ‘motor-dead’ based on an in vitro motility assay. As this residue and the region where it is located is highly conserved between muscle and non-muscle myosins of several organisms, we expected to obtain animals whose muscles were unable to contract and therefore were compromised in their movement. The result we obtained emphasised the fact that results from in vitro studies should be validated in vivo, where more complex scenarios exist.
And what about the flipside: any moments of frustration or despair?
DO Well, trying to analyse the specific role of any component of the cytokinetic contractile ring can be challenging. There is a quote from Ray Rappaport that explains it better than I can: ‘When I began working on cytokinesis, I thought I was tinkering with a beautifully made Swiss watch, but what I was really working on was an old Maine fishing boat engine: overbuilt, inefficient, never-failed and repaired by simple measures’. Additionally, myosin itself is quite a large and amazing motor and when tinkering with its gears it becomes complicated to decouple the effects on its motor versus actin-binding activities. All in all, everything involving myosin biochemistry was a challenge as we did not have any expertise.
So what next for you three after this paper?
DO I’m currently trying to capitalise on the multiple myosin strains we generated to study the interplay between muscle and non-muscle myosins in different C. elegans actomyosin-dependent contexts.
JS I am in the last year of my PhD and am currently trying to understand whether different types of myosin play a role during embryonic cytokinesis in C. elegans.
EC I am currently studying how the surrounding cortex influences the contractile ring and formin regulation throughout ring constriction.
Where will this work take the Carvalho lab?
AC There are still so many fascinating questions in the field and so studying the process of cytokinesis will continue to entertain us for a while. Our team is also moving onto exploring other cellular contexts involving actomyosin contractile networks in the powerful C. elegans system.
There are still so many fascinating questions in the field
What is the current situation for developmental and cell biology in Portugal?
AC Doing developmental and cell biology in Portugal is fun. Portugal is a beautiful and pleasant place to live, and more and more laboratories work in these fields. If only funding from the national Portuguese Foundation for Science and Technology would be more reliable and research positions more stable!
DO I think there are several very good cell and developmental biology labs in Portugal. The main challenge they face is funding and work position stability. The funding calls mostly centred on the Portuguese Foundation for Science and Technology have been quite erratic and there are very few alternatives for people trying to establish their labs in a more permanent way. Additionally, the amounts funded by national projects are not so high and this is quite an expensive field, so it is almost essential to secure funding from the European Research Council and other European sources, which are extremely competitive. As for work positions after PhD completion, the country is finally moving from a scholarship-based system to work contracts for post-docs and researchers. However, the university system is currently quite locked and most institutes, whether they are affiliated with the universities or not, have very limited possibilities to hire even the most talented PIs. This reduces my hopes that the country will succeed in maintaining and increasing the current level of its scientific community.
Finally, let’s move outside the lab – what do you like to do in your spare time in Porto?
DO Porto has become quite an amazing city over the years. There is now a whole new food, wine and craft beer scene that I love to explore while rediscovering some of the old classics. I like to walk and run around the river and seaside and when I have some free time I try to go up the river to the beautiful Douro valley where part of my family comes from. Those vineyards and amazing views bring back memories of my summer holidays as a kid. As I age, I grow more fond of them and I must confess I dream about having a vineyard of my own and producing my own wine!
EC In my spare time, I like doing different outdoor activities with my family – Porto has a wonderful beach for surfing and a large city park for my kid to ride a bike and play football.
JS I live near the beach and I like to go there and play with my beautiful baby girl. I also love to be surrounded by my friends, and we do a lot of dinners in each other’s houses.
AC Going for long walks by the beach is always reinvigorating, and going out for drinks and good food with friends is the best!


 (1 votes)
(1 votes)