Deep into the developmental origins of neural tumours with Drosophila
Posted by Caroline Dillard, on 12 July 2016
Caroline Dillard and Cédric Maurange
(Aix-Marseille University, CNRS, IBDM, Marseille)
Drosophila neural stem cells as a cancer model
Transformation of a cell into a cancer cell is a complex process along which the cell acquires hallmarks as diverse as resistance to differentiation, infinite proliferative potential, metastasis, resistance to cell death, genome instability (Hanahan and Weinberg, 2011). Moreover, tumours are usually composed of a heterogenous population of cells which may not contribute equally to the tumorigenic process. Indeed, a hierarchy exists among the tumour cells according to their potential to support tumour growth. In order to decipher the mechanisms governing the transformation process, Drosophila has been shown to be a relevant model. In 2005, Caussinus and Gonzalez elegantly demonstrated that impairing neural stem cell asymmetric division in the Drosophila larval brain triggers their amplification and the formation of large neural tumours (Caussinus and Gonzalez, 2005). Moreover, when transplanted into adult Drosophila hosts for long-term in vivo cultivation, the larval brain tumours displayed very quickly several hallmarks of cancer such as unlimited proliferative potential, genome instability or metastatic activity. In our study, we decided to use this model to determine the mechanisms contributing to the malignant transformation. Caussinus and Gonzalez impaired asymmetric neural stem cell division in a large population of neural stem cells in the Drosophila larval central nervous system (CNS). This manipulation leads to the death of the fly before it reaches adulthood. We bypassed this issue by altering only 6 neural stem cells within the larval CNS using a specific promoter. That way the Drosophila is able to survive metamorphosis and enters adulthood. In the CNS of these adults, we observe huge neural stem cell tumours expanding over and over. This observation is particularly interesting given that wild type neural stem cells possess a limited mitotic potential and terminally differentiate at the end of development, being therefore no longer present in the adult CNS. Thus, neural stem cells in tumours acquire another hallmark of cancer during metamorphosis: resistance to differentiation.
Time matters
To terminally differentiate during metamorphosis, the neural stem cell has to journey through a whole developmental program. This program is composed by sequentially expressed transcription factors known as the temporal series. They form an internal clock that governs the identity and the number of neurons generated by each neural stem cell during specific developmental windows. In Drosophila, this neural stem cell-intrinsic clock has been intensively described, particularly, during embryonic and early larval development (Isshiki et al., 2001; Maurange et al., 2008). When the progression in the temporal series is blocked (either by overexpressing or mutating one of the known temporal transcription factors), the neural stem cell stays stuck in this temporal identity and is unable to terminally differentiate during metamorphosis. As a consequence, the neural stem cell persists in the adult CNS. Given that our model of neural stem cell tumour is resistant to differentiation and that temporal series alteration prevents neural stem cells from differentiating during metamorphosis, we wondered whether temporal patterning may be altered in our tumour model.
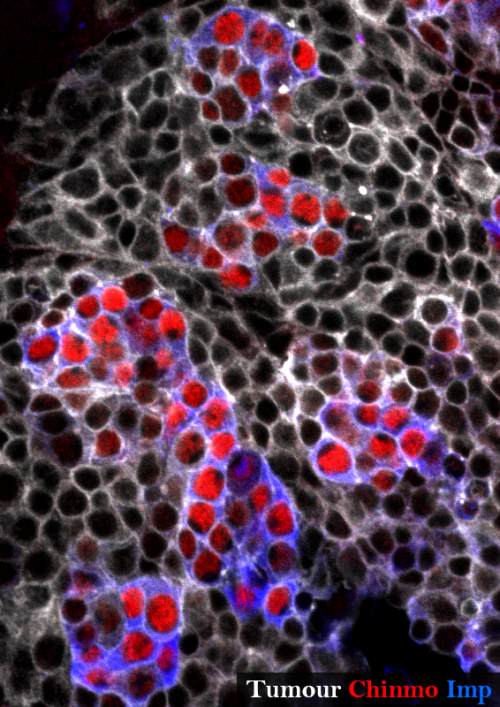
None of the known temporal transcription factors is relevantly expressed in our tumours at the end of the development demonstrating that the temporal series is not blocked in this context. Nevertheless a target of this early temporal patterning system, the ZBTB transcription factor Chinmo (Maurange et al., 2008), is expressed in a subset of tumour cells suggesting that some cells could have retained an early temporal identity.
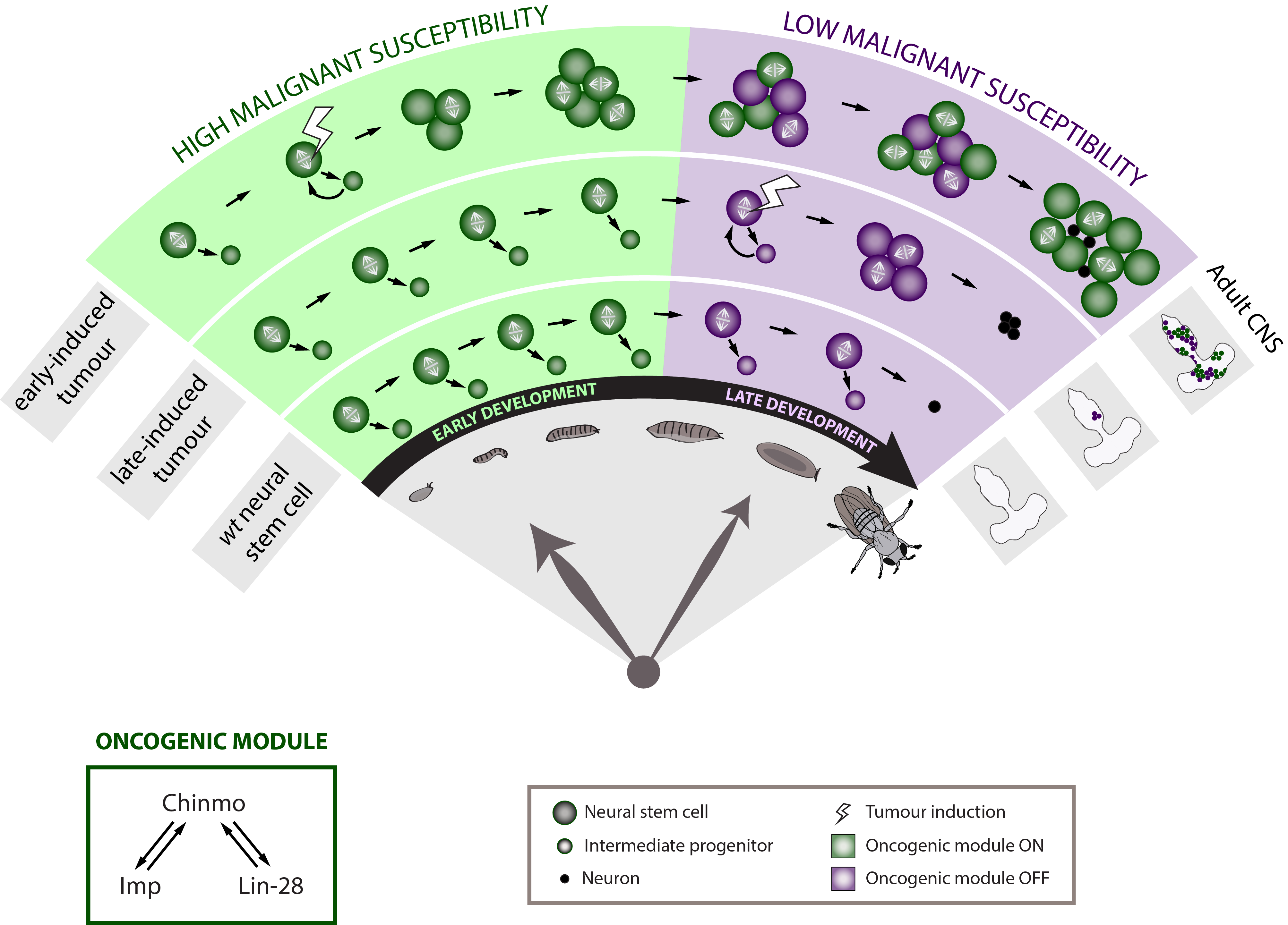
To further determine whether developmental time is a crucial component of the neural stem cell malignant transformation, we generated neural stem cell tumours at different moments along development. Tumours were initiated either during early or late larval development. Strikingly, when given the same time to grow, the late-induced tumours were largely unable to resist to terminal differentiation during metamorphosis so that few small benign tumours are found in the adult CNS whereas the early-induced tumours resisted strongly to differentiation and invaded the whole CNS. This observation demonstrates that during early development neural stem cells travel through a window of high malignant susceptibility.
Searching for the mechanisms regulating this early window of malignant susceptibility, we found that the same internal clock that limits the number of neural stem cell divisions also terminates the early window of susceptibility. Indeed, neural stem cells blocked in an early temporal identity remain prone to generate malignant tumours even when the latter are induced during late development. Thus, the temporal series must regulate genes that confer malignant susceptibility.
Malignant neural stem cells stays aberrantly young
Looking at the expression of Chinmo in the early VS late-induced tumours, we observed that Chinmo is largely expressed in the early-induced tumours but absent from the late-induced tumours. Thus, Chinmo expression correlates with malignant susceptibility in the tumorous neural stem cells. In order to establish a causality link between Chinmo’s expression in the neural stem cell and its malignant potential, we conducted gain and loss of function experiments within the early-induced tumours. We were able to show that Chinmo promotes tumour cell proliferation and resistance to differentiation. By means of a transcriptomic analysis, we identified numerous potential targets of Chinmo within the tumours. We selected the two best positive targets of Chinmo as candidates involved in the malignant process: the RNA-binding proteins Imp and Lin-28. Further genetic manipulations within the tumours allowed us to demonstrate that Imp largely contributes to the tumour growth-promoting effect of Chinmo, and Lin-28 at a lesser extent. Interestingly, Imp and Lin-28 exert also a post-transcriptional positive feedback control on Chinmo in the tumour. Thus, Chinmo, Imp and Lin-28 form an oncogenic module supporting the continuous growth of the tumour and its cancerous properties. Interestingly, we could observe that Chinmo, Imp and Lin-28 expression patterns perfectly overlap along normal development. The three genes are co-expressed in young neural stem cells but silenced in older ones. As such they define an early developmental window with specific growth and differentiation-resisting properties. However, in the developmental context, their interdependency is less clear. Together these results suggest that in our tumour model, the sub-population of cells that aberrantly maintains this oncogenic module typical from early development represents the cancer cells that propagate unlimited growth.
Relevance to paediatric cancers in humans
These results therefore demonstrate that Drosophila neural progenitors transit through a window of malignant susceptibility during early development. Moreover they reveal the timing mechanism and oncogenic module that regulate this early malignant susceptibility (Narbonne-Reveau et al., 2016). This is particularly exciting because it may explain why paediatric cancers, that are thought to originate during foetal life in humans, so rapidly become aggressive. Indeed, neural progenitors in mammals are thought to be also temporally patterned along the development although a temporal series has not been clearly identified yet (Naka et al., 2008) (Mattar et al., 2015). Moreover, Imp and Lin-28 are well conserved in mammals where they are expressed during the early development and often present in cancers (Bell et al., 2015; Carmel-Gross et al., 2016; Nielsen et al., 1999; Yang et al., 2015). Further studies would be needed to determine whether and how they promote malignant susceptibility in the early progenitors where they are expressed.
All together, this study helps identify the specific mechanisms that drive the rapid transformation of tumours with early developmental origins. In this context, work on Drosophila continues to illuminate us about the basic principles of life.
Our full paper can be viewed at here.
References
Bell, J.L., Turlapati, R., Liu, T., Schulte, J.H., and Huttelmaier, S. (2015). IGF2BP1 Harbors Prognostic Significance by Gene Gain and Diverse Expression in Neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 1285-1293.
Carmel-Gross, I., Bollag, N., Armon, L., and Urbach, A. (2016). LIN28: A Stem Cell Factor with a Key Role in Pediatric Tumor Formation. Stem Cells Dev 25, 367-377.
Caussinus, E., and Gonzalez, C. (2005). Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet 37, 1125-1129.
Isshiki, T., Pearson, B., Holbrook, S., and Doe, C.Q. (2001). Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106, 511-521.
Mattar, P., Ericson, J., Blackshaw, S., and Cayouette, M. (2015). A conserved regulatory logic controls temporal identity in mouse neural progenitors. Neuron 85, 497-504.
Maurange, C., Cheng, L., and Gould, A.P. (2008). Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell 133, 891-902.
Naka, H., Nakamura, S., Shimazaki, T., and Okano, H. (2008). Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nature neuroscience 11, 1014-1023.
Narbonne-Reveau, K., Lanet, E., Dillard, C., Foppolo, S., Chen, C.H., Parrinello, H., Rialle, S., Sokol, N.S., and Maurange, C. (2016). Neural stem cell-encoded temporal patterning delineates an early window of malignant susceptibility in Drosophila. eLife 5.
Nielsen, J., Christiansen, J., Lykke-Andersen, J., Johnsen, A.H., Wewer, U.M., and Nielsen, F.C. (1999). A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Molecular and cellular biology 19, 1262-1270.
Yang, M., Yang, S.L., Herrlinger, S., Liang, C., Dzieciatkowska, M., Hansen, K.C., Desai, R., Nagy, A., Niswander, L., Moss, E.G., et al. (2015). Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 142, 1616-1627.


 (3 votes)
(3 votes)
You might want the check out Ayeni et al. (2016) published in Development for the possible explanation.