How do pigment cells wander around?
Posted by yzhang, on 29 June 2018
The story behind melanocyte BACE2, posted by Yan Zhang and Richard White. You can read our recently published full article at Developmental Cell using this link.
Our story began six years ago when my mentor, Dr. Richard White, opened the zebrafish facility and showed me those swimming creatures. He pointed to one type with pigmented stripes and told me those are wild-type fish named AB. He caught one fish with a net and that fish very quickly jumped out of the net and escaped to a water reservoir, before I could have a closer look at her. I did not know that after that day, I would officially join a fish lab and have days and nights to observe those free swimming, free jumping animals.
That escaped fish has a stunning array of pigment patterns which is composed of three types of pigment cells: black melanophores, yellow or orange xanthophores and silvery iridophores (Parichy, 2003). While the tank next to it is filled with a transparent version, casper, where the black melanophores and the silvery iridophores are absent (White et al., 2008), some of the other fish have fuzzy pigmentation with a black tumor on the back. Those are melanomas due to uncontrolled growth of melanophores (Patton et al., 2005). I was fascinated by how diverse a pigment pattern can look like and why animals evolve them.
Animals do this for a reason. Dolphins and marlin have a darker upper surface and a white lower belly. They countershade themselves so that when seen from the top, the dark dorsal matches with deep water darkness and when seen from the bottom, the light colored belly mixes into a sunlit water above. This is one example of camouflage in front of predators. Female guppies prefer male guppies with more orange coloration, possibly because males fed on high-carotenoid diet could better reject interspecific allografts of scales and resist parasite infection, suggesting they have better immune function (Houde, 1997) – a nice example of how guppies use pigment color as a honest signal for health during sexual selection.
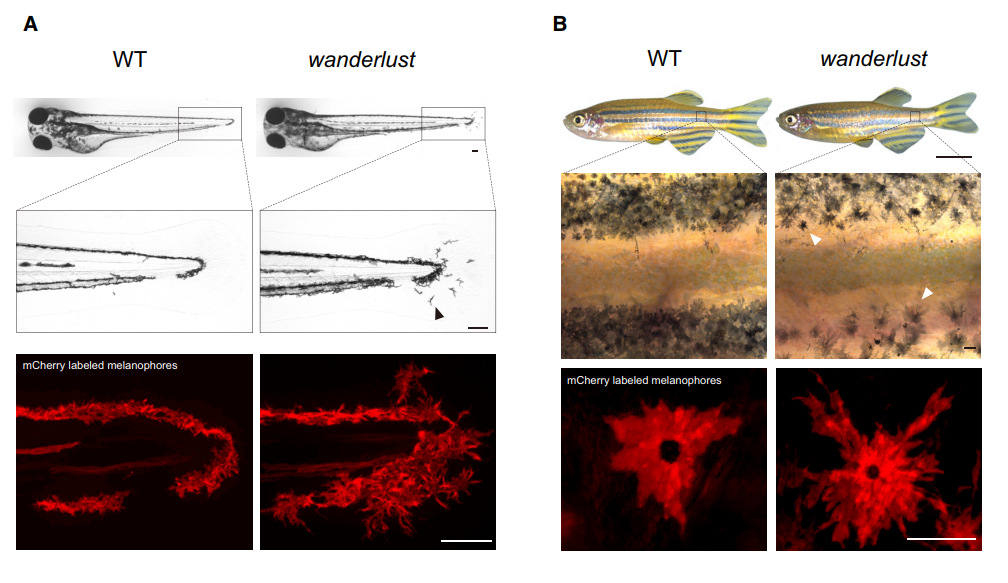
Our interests into Bace2 started when Dr. Richard White found out this gene is highly enriched in human melanoma, suggestive of oncogenic effects. We thought knocking it out would make melanomas less aggressive. But when we got zebrafish Bace2 mutants from the Sanger Center, the melanophores looked a lot “more” aggressive, an initially counterintuitive observation. It turned out this pigment mutant has super elongated melanophore cell projections, a structure named the dendrite. In mammals, melanocytes use these long dendrites to transfer melanin to neighboring keratinocytes, a process involved in tanning response to protect keratinocytes from UV-induced damage (Yamaguchi et al., 2007). Unlike mammals, zebrafish melanophores do not transfer melanin across cells, but instead traffic melanin intracellularly to modulate fish appearance (Logan et al., 2006). When melanin is aggregated around the nucleus, the fish looks lighter, and this typically occurs when fish are raised in a daylight environment. When the fish is raised in dark, melanin tends to be dispersed so that melanin covers more area and fish can match their dark-looking color with the environment. Even though zebrafish no longer use dendrites as a channel for melanin delivery, their melanophores can still be very dendritic especially when young melanophores are still actively developing. They likely use these dendrites for patterning and other ways of communicating with their neighbors. Mature melanophores lose those dendrites for unknown reasons, but not for the bace2 mutant, where their melanophores keep dendricity from embryos to adults (Figure 1).
We were intrigued by this out of control problem and sought to study why. We showed that Bace2 works during melanophore maturation, a time frame when melanophores turn on pigment genes and gain melanin. The question was how does it work? Bace2 is a cell-intrinsic sheddase which modulated cell morphology inside the melanophore lineage. We further sought to find out which protein is cleaved by Bace2 to exhibit all those phenotypes. We had no luck in the beginning. Pmel and Gpnmb are the two initial guesses as both of them are involved in melanin production and PMEL can be cut by BACE2 in mice (Rochin et al., 2013; Shimshek et al., 2016). But we soon found out those two substrates could not explain the melanophore dendricity. The research was stuck for a while. When I was even trying to grab everything I could find in our chemical room and threw them into fish to have a try, Dr. White came to me and said, why don’t we try an unbiased chemical screen?
The breakthrough came with a change in methodology. The chemical screen gave us an unexpected but fruitful hit. We found four chemicals able to convert bace2 mutant melanophores into normal looking ones, all of which converge on the same pathway-one that contains insulin, PI3K and mTOR. We realized this is something never studied before, a new PI3K/mTOR regulator that has a melanophore-specific consequence. All of the pieces came together. It turned out Bace2 itself cleaves the insulin receptor and this cleavage modulates how many functional insulin receptors are left on the cell membrane. In the mutant fish, Bace2 no longer cleaves it and melanophores have hyperactivated insulin/PI3K/mTOR which drives this uncontrolled dendricity. The driving force came from long distance, as we found that a brain-derived insulin peptide (insb) is the stimulating ligand that feeds into insulin receptor in this context.
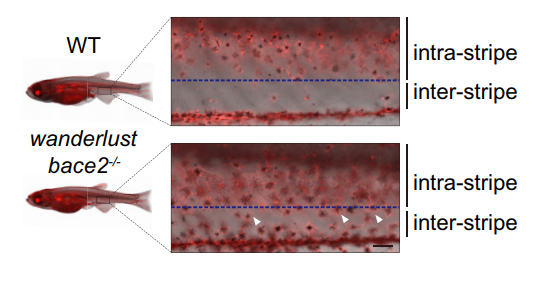
There are other consequence associated with uncontrolled dendricity: those bace2 mutant melanophores are actively differentiating, actively dividing and wandering around to ectopic locations (Figure 2). We decided to name this bace2 mutant wanderlust as those melanophores like to explore the world, travel to new sites and are free of constraints.
One thing that emerged from this research is the power of unbiased approaches to a problem. We were stuck for a while, but the screen turned out to be the most efficient and rapid way to get to the answer. It’s one of the greatest things about the zebrafish, and has allowed us to connect things – insulin and melanophores – that would have been hard to guess otherwise.
Bibliography
Houde, A. (1997). Sex, Color, and Mate Choice in Guppies. Princeton University Press.
Logan, D. W., Burn, S. F. and Jackson, I. J. (2006). Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res. 19, 206–213.
Parichy, D. M. (2003). Pigment patterns: fish in stripes and spots. Curr. Biol. 13, R947-50.
Patton, E. E., Widlund, H. R., Kutok, J. L., Kopani, K. R., Amatruda, J. F., Murphey, R. D., Berghmans, S., Mayhall, E. A., Traver, D., Fletcher, C. D. M., et al. (2005). BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254.
Rochin, L., Hurbain, I., Serneels, L., Fort, C., Watt, B., Leblanc, P., Marks, M. S., De Strooper, B., Raposo, G. and van Niel, G. (2013). BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci USA 110, 10658–10663.
Shimshek, D. R., Jacobson, L. H., Kolly, C., Zamurovic, N., Balavenkatraman, K. K., Morawiec, L., Kreutzer, R., Schelle, J., Jucker, M., Bertschi, B., et al. (2016). Pharmacological BACE1 and BACE2 inhibition induces hair depigmentation by inhibiting PMEL17 processing in mice. Sci. Rep. 6, 21917.
White, R. M., Sessa, A., Burke, C., Bowman, T., LeBlanc, J., Ceol, C., Bourque, C., Dovey, M., Goessling, W., Burns, C. E., et al. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189.
Yamaguchi, Y., Brenner, M. and Hearing, V. J. (2007). The regulation of skin pigmentation. J. Biol. Chem. 282, 27557–27561.


 (6 votes)
(6 votes)