In Development this week (Vol. 138, Issue 12)
Posted by Seema Grewal, on 24 May 2011
Here are the highlights from the current issue of Development:
How transcriptional silencing goes into reverse
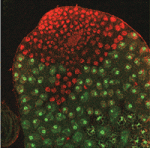
The Polycomb group (PcG) machinery silences terminal differentiation genes in stem cell lineages. Reversal of this epigenetic transcriptional silencing is implicated in the selective activation of these genes during differentiation, but little is known about the mechanism of this process. To find out more, Xin Chen, Margaret Fuller and colleagues have been examining the reversal of silencing in the Drosophila male germline stem cell (GSC) lineage. They report that developmentally regulated sequential events at promoters relieve the silenced state of the GSCs when their offspring commit to spermatocyte differentiation (see p. 2441). These sequential changes include the global downregulation of Polycomb repressive complex 2, the recruitment of hypophosphorylated RNA polymerase II to promoters, the expression and function of testis-specific homologues of TATA-binding protein-associated factors, and the function of the testis-specific meiotic arrest complex. These results provide a paradigm for how epigenetic silencing can be reversed in a gene-selective and stage-specific manner to allow the appropriate expression of terminal differentiation genes.
β-Catenin degraded by Notch in early frog development
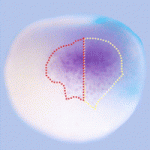
In vertebrates, the Wnt/β-catenin pathway is the core of a conserved mechanism that establishes the main body axis during early development. Now, on p. 2567, Andrés Carrasco and colleagues report that Notch restricts dorsal-anterior development in Xenopus by destabilising maternal β-catenin. The blastula chordin– and noggin-expressing centre (BCNE) is a signalling centre in early Xenopus embryos that precedes the Spemann-Mangold’s organiser and that contains brain precursors. BCNE specification depends on the dorsal accumulation of nuclear β-catenin. By injecting early embryos with Notch mRNA and morpholino constructs, the researchers show that Notch antagonises Wnt signalling by degrading β-catenin in the ventral region of the embryo. This degradation process, they report, does not require β-catenin phosphorylation by GSK3, a process that usually marks β-catenin for degradation. The researchers suggest that this interaction between Notch and β-catenin, which has not previously been recognised in vertebrates, restricts the size of the BCNE and controls the size of the brain.
Mon2 takes pole (plasm) position
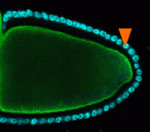
In Drosophila oocytes, the pole (germ) plasm, a specialised cytoplasm at the oocyte posterior, contains the maternal RNAs and proteins that are essential for germline and abdominal development. Akira Nakamura and co-workers now describe the role that the Golgi-endosomal protein Mon2 plays in pole plasm anchoring (see p. 2523). Pole plasm assembly begins with the transport of oskar (osk) RNA to the oocyte posterior where it is translated. Osk then stimulates endocytosis, which promotes an actin remodelling event that is essential for pole plasm anchoring. The researchers report that Mon2 interacts with Cappuccino and Spire, actin nucleators involved in osk RNA localisation in the oocyte, and promotes the accumulation of the small GTPase Rho1 at the oocyte posterior. In oocytes lacking Mon2, actin remodelling does not occur in response to Osk-induced endocytosis and pole plasm components are not correctly anchored. The researchers propose, therefore, that Mon2 is a scaffold that links Osk-induced vesicles with actin regulators to anchor the pole plasm to the oocyte cortex.
RanBPM – a scaffold for gametogenesis
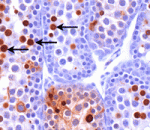
The recently identified scaffold protein RanBPM belongs to the Ran-binding protein family. Like other scaffold proteins, RanBPM interacts with numerous proteins, but what is its function? Lino Tessarollo and co-workers now report that RanBPM is essential for mouse gametogenesis (see p. 2511). Using gene targeting, the researchers show that adult RanBPM−/− mice of both genders are sterile and have atrophied gonads. They report that in male RanBPM−/− mice the testes develop normally for one week postnatally but that there is then a marked decrease in spermatogonia proliferation. The first wave of spermatogenesis, they report, is characterised by spermatocyte apoptosis towards the end of prophase I. Moreover, experiments in chimeric mice indicate that RanBPM acts in a cell-autonomous way in male germ cells. Finally, they show that fertility in female RanBPM−/− mice is compromised because of germ cell depletion at the end of prophase I. Thus, mammalian RanBPM plays a crucial role in both spermatogenesis and oogenesis.
Gli-ful insights into Hedgehog signalling conservation
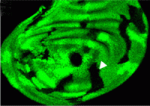
Hedgehog (Hh) signalling controls cell fates and cell proliferation in many animals by regulating gene transcription. Some parts of the Hh pathway, including the final transcriptional effectors, are highly conserved. Thus, in flies and mammals, Hh signalling activates full-length Cubitus interruptus (Ci) and Gli family transcription factors, respectively, and prevents Ci/Gli proteolytic processing to repressor forms. But, are the molecules that regulate Ci/Gli protein activities similarly conserved? Steven Marks and Daniel Kalderon address this contentious topic on p. 2533 by investigating the regulation of mammalian Gli proteins in Drosophila cells. They show that the fly kinesin-family protein Costal 2 (Cos2), which directs Ci processing in Drosophila, binds to three regions of the transcriptional activator Gli1, just as for Ci, and that Cos2 silences mammalian Gli1 in Drosophila cells in an Hh-regulated manner. They also show that Gli regulation by protein kinase A is conserved between flies and mammals. Together, these results reveal a greater degree of Hh pathway conservation than was previously recognised.
POU proteins: ancient stem cell regulators
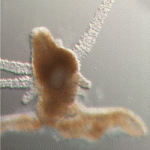
What is the evolutionary origin of pluripotent stem cells? On p. 2429, Uri Frank and colleagues provide new insights into this longstanding question by studying the marine cnidarian Hydractinia echinata. In mammals, pluripotent stem cells are limited to early embryos where they are induced and maintained by the POU domain protein Oct4 and certain other key transcription factors. By contrast, clonal invertebrates such as H. echinata possess pluripotent stem cells throughout their life. Here, Frank and colleagues report that Polynem (Pln), a putative homologue of Oct4, is expressed in the embryonic and adult stem cells of H. echinata and that ectopic expression of Pln in epithelial cells induces stem cell neoplasms and loss of epithelial tissue. Neoplasm cells, they report, downregulate expression of the Pln transgene but express the endogenous Pln gene and other cnidarian stem cell markers. Conversely, Pln downregulation by RNAi leads to differentiation of adult stem cells. Together, these results suggest that POU proteins are conserved regulators of stem cells.
Plus…
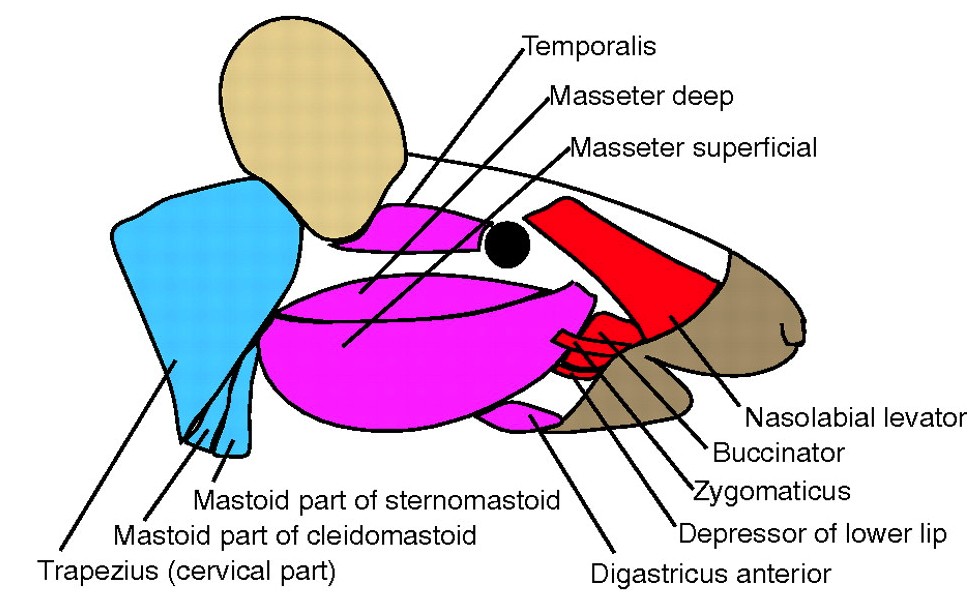
Recent evidence suggests that craniofacial muscles are evolutionarily, morphologically and molecularly distinct from those of the trunk. Here, Sambasivan, Kuratani and Tajbakhsh review these studies and discuss the molecular basis of craniofacial muscle development.
See the Review article on p. 2401


 (No Ratings Yet)
(No Ratings Yet)