In Development this week (Vol. 138, Issue 2)
Posted by Seema Grewal, on 21 December 2010
Boning up on stem cell Igf2-P2 function
The insulin-like growth factor (IGF)/insulin signalling pathway regulates cell proliferation, differentiation, aging and life span. During embryonic development, transcription of the mouse and human Igf2 gene is tightly regulated by four alternative promoters whose specific roles are unclear. Now, Sylvie Nathalie Hardouin and colleagues reveal that the transcriptional activity of one of these promoters, Igf2-P2, regulates mesenchymal stem cell differentiation and osteogenesis in mice (see p. 203). The researchers show that Igf2-P2 loss-of-function mice, in which a lacZ-neo cassette replaces the P2-driven transcriptional unit of Igf2, have short, thin, poorly mineralised bones and exhibit altered bone remodelling. These abnormalities are associated with decreased numbers of embryonic mesenchymal chondroprogenitors, adult mesenchymal stem cells and osteoprogenitors. Together, these and other results support a model in which the transcriptional activity of the Igf2-P2 promoter regulates the fate of mesenchymal progenitors during bone development and adult bone remodelling, and regulates osteogenesis through its effects on both osteoprogenitors and their microenvironment.
X inactivation: from imprinted to random
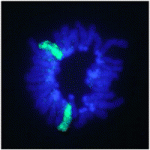
In female mammals, one X chromosome is epigenetically silenced in adult cells by the process of X inactivation (Xi). However, in the pluripotent epiblast cells of the preimplantation mouse embryo, both X chromosomes are active and Xi of the paternal or maternal X occurs at random shortly after implantation (random Xi). By contrast, in very early mouse embryos (and in extra-embryonic lineages), the paternal X chromosome is selectively inactivated (imprinted Xi). So when exactly does the mode of Xi change from imprinted to random during development? On p. 197, Hitoshi Niwa and colleagues examine Xi during the differentiation of inner cell mass (ICM)-derived female mouse embryonic stem (ES) cells. The researchers use forced expression of Cdx2 and Gata6 to induce ES cell differentiation toward trophectoderm (TE) and primitive endoderm (PrE), respectively. They report that random Xi occurs in both TE and PrE cells and in the TE of cloned embryos derived from female ES cells, suggesting that all marks for imprinted Xi must be erased by the time the ICM forms.
Muscle building: a connective tissue workout
Muscle and its connective tissue are intimately linked during embryogenesis and adult life. Thus, interactions between these tissues might be crucial for their development. To date, the lack of molecular markers for connective tissue fibroblasts has hindered the study of these potentially important interactions, but now, on p. 371, Gabrielle Kardon and colleagues identify the transcription factor Tcf4 as a marker for connective tissue fibroblasts and reveal that connective tissue is an important regulator of myogenesis. By making Tcf4GFPCre mice, which allow genetic manipulation of connective tissue fibroblasts, they show that these fibroblasts regulate both muscle fibre type and maturation. In addition, the researchers unexpectedly discover that low levels of Tcf4 in myogenic cells promote the overall maturation of muscle fibre type. These and other data identify novel extrinsic and intrinsic mechanisms that regulate myogenesis and show for the first time that connective tissue is a vital component of the niche that controls muscle development.
Mitochondrial pathway central to fly apoptosis
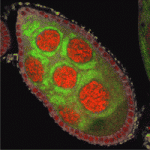
Apoptosis (programmed cell death) is essential for development and tissue maintenance in many organisms. In mammals and C. elegans, Bcl-2 family proteins facilitate apoptosis by regulating mitochondrial dynamics but do they play a similar role during apoptosis in Drosophila? According to Kimberly McCall and co-workers, the answer to this question is yes in the Drosophila ovary (see p. 327). During mid-oogenesis in flies, apoptosis is induced in some of the egg chambers in the ovary when nutrients are scarce. The researchers show that, during this event, the mitochondrial networks of ovarian nurse cells undergo extensive remodelling, cluster formation and cluster engulfment by somatic follicle cells. These mitochondrial dynamics, they report, are dependent on caspases, the Bcl-2 family, the mitochondrial fission and fusion machinery and the autophagic machinery. Furthermore, cell death in the ovary is defective in Bcl-2 family mutants. Thus, the researchers conclude, Bcl-2 family proteins do play a major role in controlling both mitochondrial dynamics and cell death in the Drosophila ovary.
Retinal cell fates largely left to chance
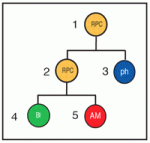
Classic experiments in invertebrates suggest that stereotypic patterns of cell division generate specific cell types during development, but the extent to which stereotypic lineages play a part in the developing vertebrate CNS is an open question. Now, Michel Cayouette and co-workers report that stochasticity plays a major role in cell fate decisions in the developing rat retina (see p. 227). In vivo cell-lineage tracing studies show that vertebrate retinal progenitor cells (RPCs) yield retinal clones of varying size and cellular composition. Whether this variability reflects distinct but reproducible lineages among many different RPCs or stochastic fate decisions within a population of more equivalent RPCs is unclear. To find out, the researchers use videomicroscopy to follow the lineages of rat RPCs cultured at clonal density. Their analysis of the reconstructed lineages indicates that fixed probabilities determine the decision of the RPCs to multiply or differentiate. Thus, stochasticity plays a major part in the development of the retina and possibly also of other parts of the vertebrate CNS.
Worming out organiser evolution
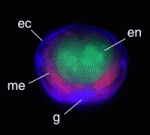
Axial organisers, embryonic regions that induce cell fate and establish body axes during development, have been identified in various metazoans but their evolutionary origins and conservation of function remain unclear. The presence of an axial organiser in annelids (ringed worms) has not previously been confirmed, but now Takashi Shimizu and colleagues provide direct evidence that, in Tubifex tubifex annelids, descendants of a single blastomere of 4-cell embryos can function as axial organisers (see p. 283). The first two cleavages of T. tubifex embryos generate four macromeres (A-D) that subsequently divide to generate micromeres. The researchers show that ablation of the D macromere descendants 2d and 4d can inhibit axial development. Co-transplantation of 2d and 4d into ectopic positions, they report, induces secondary axis formation in host embryos; in these axes, neurectoderm and mesoderm derive from the transplanted micromeres, whereas the endoderm derives from the induced host. These studies identify D quadrant micromeres as annelid axial organisers, informing future studies of axial organiser conservation and evolution.
Plus…
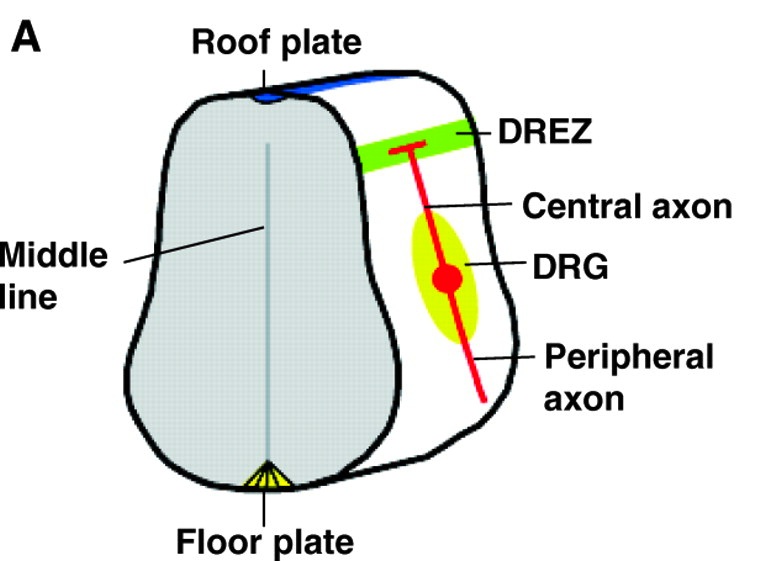
During nervous system development, axon branching allows elaborate synaptic connections to form. Recent advances, reviewed by Daniel Gibson and Le Ma, identify how various axon branching morphologies develop and the common principles that regulate them.
See the Review article on p. 183 of this issue.


 (No Ratings Yet)
(No Ratings Yet)