Mechanisms for asymmetric heart morphogenesis: About Nodal and tissue intrinsic forces
Posted by j.bakkers, on 11 December 2013
Although we all appear symmetrical from the outside, the organization of our internal organs and organ structure are highly asymmetric. Proper asymmetric positioning and patterning of our organs is very important for correct function, and loss of this asymmetry during organ formation can lead to a variety of serious congenital diseases. Our recent study identifies a new mechanism by which the heart forms an asymmetric structure during embryonic development.
Thus far it was thought that all organs use the same mechanism to develop this left-right asymmetry by responding to the TGF-β-related growth factor Nodal, which is expressed on the left side of the developing vertebrate embryo. Using forward genetic screening in zebrafish for mutants defective in organ laterality we identified the straightforward mutant. Interestingly the straightforward mutant embryos exhibited complete randomization of brain and the visceral organ laterality, while preferential direction of heart looping was maintained. Positional cloning of the mutation underlying the organ laterality defect revealed a loss-of-function mutation in the asymmetrically expressed zebrafish Nodal-related gene southpaw (spaw).
In vertebrates, including zebrafish, the heart starts out as a linear tube that bends to the right and loops asymmetrically to form the mature functional heart. In zebrafish embryos the linear heart tube is also positioned asymmetrically with respect to the midline with the future venous pole positioned ventral to the left eye. In spaw mutant embryos this initial leftward positioning of the heart (named cardiac jogging) is compromised resulting in a midline positioned heart. Despite this cardiac jogging defect in spaw mutant embryos, the majority of the hearts bend to the right forming a normal dextral heart loop. We therefore concluded that (1) Spaw activity is required for cardiac jogging, but cardiac jogging is dispensable for asymmetric heart looping and (2) Spaw is not the only regulator of asymmetric heart looping. Since there are three Nodal-related genes expressed in the zebrafish embryo we examined whether these other Nodal-related proteins could provide asymmetry information to the heart during heart looping and thereby could compensate for the loss of Spaw activity. However, from our experiments we concluded that the other Nodal-related genes are absent from spaw mutant embryos at embryonic stages relevant to heart lateralization. In addition we observed preferred dextral looping in spaw mutant embryos in which Nodal signaling was blocked either chemically or genetically. From these results we concluded that in the absence of Nodal signaling, dextral heart looping in zebrafish embryos is regulated by an alternative mechanism.
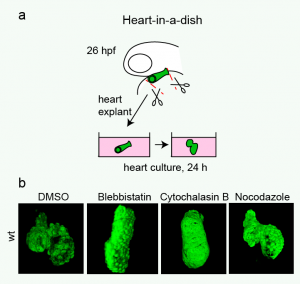 Heart looping in a dish. (a) Cartoon showing the procedure of culturing embryonic heart tubes. Linear heart tubes were dissected from tg(myl7:GFP)-positive embryos at 26 hpf, placed into culture medium and incubated further. (b) Images of explanted heart tubes after 24 hours in culture. While control hearts completed dextral-looping after a 24 hours culturing period, treatment of explanted heart tubes with either a myosin II inhibitor (Blebbistatin) or an inhibitor of actin polymerization (Cytochalasin B) prevented normal heart looping. Reproduced with kind permission from Nature Communications.
Heart looping in a dish. (a) Cartoon showing the procedure of culturing embryonic heart tubes. Linear heart tubes were dissected from tg(myl7:GFP)-positive embryos at 26 hpf, placed into culture medium and incubated further. (b) Images of explanted heart tubes after 24 hours in culture. While control hearts completed dextral-looping after a 24 hours culturing period, treatment of explanted heart tubes with either a myosin II inhibitor (Blebbistatin) or an inhibitor of actin polymerization (Cytochalasin B) prevented normal heart looping. Reproduced with kind permission from Nature Communications.
Significantly, we found that this asymmetric development of the heart is mostly independent from external factors or forces from the rest of the embryo. We developed a heart-in-a-dish system that allowed us to culture linear heart tubes dissected from the embryo in a dish. Linear heart tubes dissected from the embryo retain their ability to loop, and heart looping occurred predominantly in the correct dextral direction. The heart-in-a dish system allowed us to test the effect of various drugs on directional heart looping and we found that inhibiting actin polymerization or non-muscle myosin activity disrupted the looping process. From these results we concluded that the asymmetric development of the heart is regulated by different mechanisms than those governing brain and visceral organ asymmetry – specifically, heart looping is a tissue intrinsic process that requires actomyosin activity.
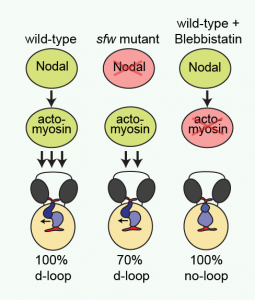 Cartoon illustrating proposed regulation of dextral heart looping by Nodal and actomyosin. In a wild-type situation, combined Nodal signaling and actomyosin activity provide a robust mechanism driving dextral heart looping. In the absence of Nodal signaling, actomyosin activity is sufficient to promote preferential dextral heart looping. In the absence of actomyosin activity, Nodal signaling is not sufficient to promote dextral heart looping. Reproduced with kind permission from Nature Communications.
Cartoon illustrating proposed regulation of dextral heart looping by Nodal and actomyosin. In a wild-type situation, combined Nodal signaling and actomyosin activity provide a robust mechanism driving dextral heart looping. In the absence of Nodal signaling, actomyosin activity is sufficient to promote preferential dextral heart looping. In the absence of actomyosin activity, Nodal signaling is not sufficient to promote dextral heart looping. Reproduced with kind permission from Nature Communications.
These findings are interesting since in Drosophila, which lack Nodal genes in their genome, asymmetric organ formation is controlled by tissue- and cell-intrinsic mechanisms that require specific components of the cytoskeleton. Similarly, cultured mammalian cells show phenotype-specific left–right asymmetry that is controlled by the cells cytoskeleton. Our findings suggest now that such an intrinsic mechanisms is also important to regulate asymmetric heart development in zebrafish. Indeed, in ancestral vertebrate organisms that exhibit asymmetric embryonic Nodal expression, accompanied by brain and gut asymmetries, no heart asymmetries are observed in the developing embryo. This suggests that ancestrally the heart did not respond to asymmetric Nodal signaling and it is interesting to speculate that heart asymmetry developed independent of asymmetric Nodal.
reference:
Noël ES, Verhoeven M, Lagendijk AK, Tessadori F, Smith K, Choorapoikayil S, den Hertog J and Bakkers J. (2013) A Nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat Commun. 2013 Nov 11;4:2754


 (4 votes)
(4 votes)