Senescent cell rejuvenation – you(r cells) are never too old for pluripotency!
Posted by Sasha Terashima, on 3 December 2011
In 2007, a group let by Takahashi and Yamanaka from Kyoto University successfully generated pluripotent cells from human adult fibroblasts. They were able to induce a pluripotent state in differentiated cells by introducing four transcription factors, OCT4, SOX2, c-MYC, and KLF4 by retroviral infection, hence the name “induced pluripotent stem cells (iPSCs).” Although the mechanism of how these factors induced pluripotency in somatic cells is not completely understood, it is clear that the endogenous pluripotency genes OCT4, SOX2 and NANOG were activated and, in turn, re-activated the autoregulatory loop that could maintain the pluripotent state independent of the transgenes. iPS cells showed many characteristics of human embryonic stem cells (hESCs) such as expression of pluripotency markers, reactivation of telomerase and the ability to form teratomas, demonstrating a potential to redifferentiate into descendants of all three embryonic lineages.
However, follow-up studies suggested that the reprogramming of iPS cells was incomplete. Some epigenetic imprinting remained, the telomeres length was not fully restored, and the descendants of these cells entered senescence prematurely. Additionally, it was reported that cells from older donors were difficult to convert to iPS cells.
As people age the number of cells that are senescent increases. Senescence is defined as an irreversible cell proliferation arrest and occurs in response to various stresses, including activation of oncogenes, shortened telomeres, DNA damage, oxidative stress and mitochondrial dysfunction. Common features of senescence include activation of the p53/p21 and p16/pRb pathways and formation of senescence-associated heterochromatic foci (SAHF).
Conversion of somatic cells to iPS cells occurs at very low frequency in any given cell population, but because older individuals have a higher number of senescent cells it has proved to be difficult to convert cells from older-aged donors. In an effort to overcome this barrier some researchers tried an alternative four-factor combination, substituting NANOG and LIN28 for c-MYC and KLF4, but without much improvement. Researchers began to wonder whether cellular aging was a barrier to iPS cell conversion.
In a recent paper published the November issue of Genes in Development, entitled “Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state,” Lapasset and colleagues from the Institute of Functional Genomics in France report that they have overcome this barrier and generated iPS cells from human donors as old as 101 years. What’s more, the converted cells showed no signs of premature aging and appeared “rejuvenated” – iPS cells converted from nearly senescent donor cells regained their replicative potential and, when re-differentiated to fibroblasts, by all accounts resembled young proliferative cells.
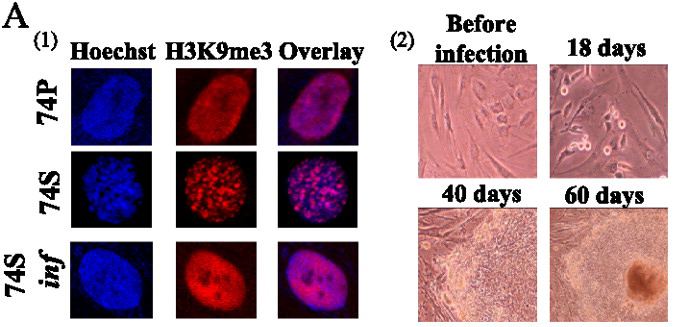
The key was to use six transcription factors, not four, combining OCT4, SOX2, c-MYC, KLF4, NANOG and LIN28. Initially, they took fibroblasts from a 74-year-old man and induced them into replicative senescence by serial passaging. Senescence was confirmed by FACS analysis showing cell cycle arrest, increase in molecular markers characteristic of senescence, and formation of SAHF.
The six transcription factors were introduced by lentiviral infection. A week after infection, the SAHF disappeared (see figure above, left panel) and after 40 days colonies appeared that looked like hES cells (see figure above, right panel). Lapasset et al. examined individual clones and found that endogenous pluripotency gene expression was activated and the promoters of OCT4 and NANOG, which are usually heavily methylated in differentiated cells, were demethylated in the newly converted iPS cells. Individual clones were able to differentiate into cells expressing markers of all three germ layers as well as form teratomas with organ-like structures typical of all three embryonic lineages.
The authors then repeated this procedure with cells from donors 92, 94, 96 and 101 years of age and again were successful in generating iPS cells with the same efficiency, making these the oldest human donors so far whose cells were reprogrammed for pluripotency.
They extensively tested whether the iPS cells retained marks of aging similar to cells they originated from. They found that unlike parental cells, p16 and p21 expression in iPS cells was downregulated, similar to hES cells. Additionally, telomere length was restored and maintained after numerous population doublings. Because previous reports using the 4-factor induction method reported that iPS cells induced from aged donor cells have chromosomal abnormalities the authors examined the karyotypes of the iPS, but found that in all cases they were normal.
They went on to compare the transcriptomes of the iPS cells with those of the hES cells and the parental cell types. The result was that the iPS cells gene expression profile had much more in common with hES cells and very little with the parental cells.
The final question they addressed was whether reprogramming of senescent cells and cells from long-lived donors to a pluripotent state leads to the production of “young” cells upon redifferentiation. Previous studies of fibroblasts derived from iPS cells showed that they have limited replicative potential and entered senescence early. In this study, when the iPS cells derived from 74-year-old and 96-year-old donors were redifferentiated to fibroblasts their rate of proliferation was similar to young proliferative fibroblasts. The cells had regained replicative potential and were able to go through additional 60 population doublings before re-entering senescence, in contract to the sencescent cells they were derived from, which were no longer capable of replicating.
Transcriptome analysis of the newly differentiated fibroblasts showed that they resembled young proliferative embryonic fibroblasts derived from hES cells rather than their parental cell types. They also had less oxidative stress and better mitochondrial function than the parental cells. The authors concluded that the cells were “rejuvenated” as a result of reprogramming through the pluripotent state.
This paper represents a significant advance in the field of iPS cells, demonstrating that cellular aging is not a barrier to generating pluripotent cells, bringing us one step closer to cell-based therapies for aged patients.
![]()
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, & Lemaitre JM (2011). Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes & development, 25 (21), 2248-53 PMID: 22056670


 (3 votes)
(3 votes)
kinda curious about the mechanism of those two added genes