The people behind the papers – Adrian Danescu, Lisanne Rens and Joy Richman
Posted by the Node Interviews, on 2 August 2021
This interview, the 98th in our series, was published in Development earlier this year.
During vertebrate face development, bilateral streams of neural crest cells migrate from the neural tube to give rise to the facial prominences. A new study in Development combines high-resolution live imaging of chick facial development with a mathematical examination of cell behaviour to understand the dynamics of facial symmetry. We caught up with Adrian Danescu, Lisanne Rens and corresponding author Joy Richman (Professor and Director of the Pediatric Dentistry Graduate Program in the University of British Columbia in Vancouver, Canada) to find out more about the work.

Joy, can you give us your scientific biography and the questions your lab is trying to answer?
JR: I was originally trained as a dentist and then specialized in paediatric dentistry. It was while I was doing my specialty training that I first encountered the field of developmental biology. The tooth development project with Ed Kollar was so enjoyable that I went on to do a PhD in craniofacial development with Cheryl Tickle at UCL. She was an outstanding mentor and although your audience will be very familiar with her pioneering work on limb development, she also had three students that worked on the face and I was one of them. After that experience in London, the chicken was my main model organism until the last 15 years when I started also working on non-avian reptiles (lizards, snakes, turtles). My group has made discoveries concerning the molecular mechanisms of facial morphogenesis; however, all our previous work was done with static analysis. This is our first foray into time-lapse imaging to describe cellular behaviours in real time. We certainly did not anticipate the striking choreography of cell movements in the face way back when we started this project.
Adrian, how did you come to join Joy’s lab and what drives your research today?
AD: Like Joy, I also trained as a dentist, in Romania, and then came to Canada to take an MSc degree. I was interested in embryology and I decided to enrol in a PhD project in a lab that focused on craniofacial development – Joy’s lab at UBC was a perfect fit for me. My project involved facial birth defects, and their proper study required a suitable model. The lab was well versed in avian techniques and for me it was essential to learn quickly all the technical aspects necessary for the ongoing project. My entire PhD was a dynamic journey, with lots of opportunities for exciting projects and networking.
My main accomplishment in the lab was to develop a system to observe the movement of mesenchymal cells within the face during the early stages of development, prior to lip fusion, with high-resolution microscopy. This took several years of optimization and painstaking attention to subtle things such as finding a way to label individual cells. At the beginning of the project, I collaborated with an expert in lipid nanoparticles so I could deliver plasmids to the chicken face without electroporation. I now recognize the practical applications of these in vivo transfection methods after the implementation of the same technology to make COVID-19 mRNA vaccines. After gathering detailed tracking data, we started to collaborate with Leah Edelstein-Keshet and her postdoc Lisanne Rens from the department of Mathematics at UBC. They came at the data from a different angle and thanks to their insights the paper reached a deeper significance.
Lisanne, what is your research history and how did you come to be involved in this project?
LR: I am from the Netherlands, which is also where I obtained my PhD in mathematical and computational biology (from Leiden University). I typically use mathematical modelling techniques to describe processes in development, such as cell migration, angiogenesis, branching morphogenesis. At the start of this project, I was a postdoc at the mathematics department at UBC, working with my advisor Leah Edelstein-Keshet. I became involved in this project because Joy and her group were looking to quantify their data and understand it better. They got in contact with Leah, and I was very excited to be involved because I was already familiar with suitable methods, which I previously used to quantify simulated data. Just to be able to work with experimental biologists is a great opportunity. After our first meeting, Adrian sent me their data, and then it all started.
How has your research been affected by the COVID-19 pandemic?
AD & JR: I think we were fortunate enough that most of our experiments and analyses were completed before COVID-19 changed our way of working. There were only a few experiments left to finalize the paper and we planned them right after the restrictions were lifted. Communication via online platforms was convenient enough, especially for being able to bring other people from various places to participate in our discussions. However, the lab closure delayed the time we initially planned to finish the paper.
LR: For me, as a computational biologist, working at home is not as big of a deal compared to wet-lab biologists. However, like with any person, working from home comes with challenges. I find it lonelier, and it’s harder to keep being motivated. Also, with school closures, the kids are around.
What is the theory of developmental instability, and how does it relate to craniofacial abnormalities?
AD, LR & JR: Developmental instability refers to the range of fluctuations during development that can be usually compensated by various mechanisms to maintain normal development. There are both genetic and environmental factors at play, but our focus was to find evidence of instability at the cellular level. The theory is that all embryos will have some degree of developmental instability and, in the majority of cases, normal morphogenesis occurs. However, a slight increase in instability may be enough to lead to congenital malformations such as cleft lip. This instability is not only particular to the craniofacial area, but it may also concern all organs. One of the ways to measure developmental instability in other systems is to look at symmetry. Since we had dissected the midline of the face, we were able to compare the left versus right side. Through fruitful discussions with Lisanne and Leah Edelstein-Keshet, the idea to map the data back onto a grid was developed. In that way, we could compare directly each grid reference point in the equivalent anatomical location.
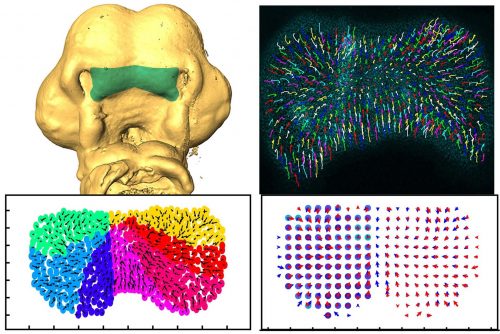
Can you give us the key results of the paper in a paragraph?
AD, LR & JR: In this study, we wanted to understand the striking shape changes in early facial development prior to lip fusion. We turned to high-resolution live imaging in order to globally track hundreds of individual mesenchymal cells across the frontonasal mass. First, we discovered that mesenchymal cells moved; second, the movements alternated between states of order and disorder; third, clustering algorithms revealed that the movements are coordinated over large distances; and fourth, by interpolating the data we found symmetry that also fluctuates over time. We then used this interpolated data to map patterns of divergence and convergence that are again cyclical. We showed that all these cell behaviours are dependent on the actomyosin network. One of the most interesting observations made through mathematical modelling was the correlation between the switches in direction of movement with transitions from states of order to disorder.
What did the mathematical analysis of cell behaviours reveal that simple observation could not?
AD, LR & JR: First, the analysis confirmed some of the patterns we thought we were seeing. For instance, after quantification, the symmetry we noticed by eye became very clear, and the loss of symmetry was tremendous in the knockout tissue. We also noticed changes in the direction of the cells, but the pattern was not clear. This is where a divergence analysis helped. It revealed bands of convergence and divergence, and how they changed over time. Our velocity correlation analysis revealed the spatial distance across which cells are seemingly able to communicate. By clustering algorithms, we identified the spatial regions of coordinated motion, which was not possible to do by hand.
Furthermore, the order/disorder and the K-means clustering analyses revealed fluctuations of cell behaviour at a smaller scale, indicating developmental instability during normal midface development. Modelling also helped us discover the rapid switches of cell direction between divergence and convergence that happens within 20 minutes. The overall symmetry and periodicity during midface development were identified by the mathematical modelling as well.
The cell movements you observe are often symmetrical – what might explain this coordination of behaviour over such a long range?
AD, LR & JR: Several pathways, such as WNTs, BMPs, SHH, FGFs and so on, are at play during midface morphogenesis, and they may have a role in regional or more global coordination of cell movement. Furthermore, the forces generated through the extracellular matrix may contribute to these movements as well, due to cells being connected as a network. We identified one potential candidate to be WNT5A signal, with its expression overlapping the band of divergence and convergence that we identified. Our observations will pave the way for future investigations into the molecules that play an essential role in either buffering against instability or promoting the fluctuations. Ultimately, gene pathways associated with increased risk of clefting will be tested in this system.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
AD: The first moment that stuck with me was observing how dissected faces grew and developed normally in a culture environment. I realized the advantage of this system, being suitable for direct observation under the microscope, in contrast with the side positioning that can be accessed in a developing embryo inside the egg. However, this was just the beginning of a long and laborious process to set up a series of methods to pursue my project.
LR: I was most surprised that we were able to link changes in divergence/convergence and coordinated/uncoordinated motion of the cells. Both of these quantities varied in time in a similar periodic fashion. This bears the question which one of those precedes the other, or how they are linked.
And what about the flipside: any moments of frustration or despair?
AD: There were countless moments of frustration, as always happens in research. Along the years, I learned to manage and embrace them as a way to grow. Learning the dissection techniques, optimizing the imaging setup for better clarity and stability of the culture during imaging, finding the right people to contribute to the project were all challenging, but what matters in the end is never, ever give up.
LR: For me, luckily not as many frustrations as with other projects I’ve done! I think the biggest challenge was how to interpret what the quantification of the data was telling us: what are the possible underlying mechanisms?
What next for you two after this paper?
AD: I graduated from both the PhD and the clinical program in Orthodontics last year. I have started to look for opportunities to find my way back into research. I intend to find the optimal way to work as a clinician and dedicate time for research. I expect this process to take longer as we are in the third wave of COVID-19 now in Canada, and there is still a lot of concern and uncertainty for the near future.
LR: I recently started a tenure track position at the TU Delft in my home country. I work in the mathematical physics group, continuing my line of research into mathematical modelling of cell and tissue biology.
Where will this story take the Richman lab?
JR: I would love to go further into the environmental influences that most affect developmental instability. I will expand the work to test specific pathways as mentioned above. In the current paper, we employed a global block on small GTPase signalling, but in the future we will be more specific.
I would love to go further into the environmental influences that most affect developmental instability.
Finally, let’s move outside the lab – what do you like to do in your spare time?
AD: I recently moved to Ontario after many great years in beautiful Vancouver. I generally try to allocate time for cooking, reading, travelling, doing outside activities, and spending time with my wife. I am learning that Ontario is fantastic for outdoor explorations with numerous lakes and parks. The summer is coming, and hopefully we will be able to enjoy a more normal summer after a tough year with so many lockdowns.
LR: Classical ballet is a big passion of mine, so during my time in Vancouver, I was practicing ballet downtown for two hours per week. Now in the Netherlands, I am back at my old ballet school. I also have two young kids who I spend most of my spare time with.
JR: I enjoy walking along the beach or in one of the many lovely urban forests near the UBC campus with my dog. I also am a passionate Masters swimmer and hope to return to club swimming soon. Right now, the pool is shut because of COVID-19. I also have really enjoyed spending lots of quality time with my two children in their twenties who came home to shelter during the pandemic. Oh yes, I have also kept my sour dough starter alive for a year now. Being a scientist helps!


 (No Ratings Yet)
(No Ratings Yet)