The people behind the papers – Caroline Johnson and Troy Ghashghaei
Posted by the Node Interviews, on 15 October 2020
This interview, the 79th in our series, was published in Development earlier this year.
Cortical development involves a switch from the self-amplification of stem cells to the generation of neuron and glia by progenitors. A new paper in Development investigates the molecular control of mitosis in these two stages, using simultaneous labelling and gene knockout in clones in the developing mouse brain. We caught up the paper’s two authors Caroline Johnson and her supervisor Troy Ghashghaei, Professor of Neurobiology at the College of Veterinary Medicine at North Carolina State University, to find out more.

Troy, can you give us your scientific biography and the questions your lab is trying to answer?
TG I did my graduate work mapping limbic circuits, focusing on connections between the basal forebrain/amygdaloid nuclei and the prefrontal cortices of macaque monkeys. Some of the results raised a number of developmental questions, which led me to pursue a postdoc in developmental neurobiology. Therefore, I did my postdoc work on adult neurogenesis and cortical development, which we have continued in my own lab since 2006. I am now a professor and my lab is currently focused on two main projects: (1) how neural stem cells undergo developmental transitions during distinct time points, and how perturbations of these transitions impact proliferation and differentiation in the forebrain and cortex; (2) how ependymal cells are formed from embryonic neural stem cells and how these fascinating and poorly studied cells regulate forebrain homeostasis during adulthood and aging. We use a genetic approach in understanding the functions of various factors, and employ interdisciplinary tools to identify, characterize and test various phenotypes in the two projects.
Caroline, how did you come to work with Troy and what drives your research today?
CJ When I was an undergraduate at UNC Chapel Hill, I volunteered with the veterinarians at the Duke Lemur Center, and worked at Duke studying the diversity of mouse lemur species. I then worked as a technician at Harvard, which sparked my interest in neuroscience research. When I was looking for graduate programmes, I wanted to move back to North Carolina because there are a lot of great research opportunities here and I could be closer to my family. I was also potentially interested in veterinary medicine research, which led me to look at graduate programmes with the veterinary school at NC State University [NC State]. When I heard about the projects in developmental neuroscience research occurring in Dr Ghashghaei’s lab, I contacted him and enrolled in the Comparative Biomedical Sciences programme. Cortical development is an amazing field. The sensitivity of the cortex to disruptions in cell division and the paradigm of its development permits us to ask questions about basic development, comparative development and cell proliferation, and gives us greater insight into how this fascinating structure has developed and evolved.
How did you come to study Sp2 and what was the main question your current paper was aiming to answer?
TG My colleague Jonathan Horowitz and his lab were researching the function of Sp2 in cancer cells when I first arrived at NC State. Sp family members have a fascinating genomic organization and they are all involved with important aspects of organismal development. When we began this project, Sp2 was the least studied family member. In collaboration with the Horowitz lab we found that Sp2 is highly expressed in neurogenic regions of the postnatal and embryonic mouse forebrain. This led a previous graduate student in the lab, Huixuan Liang, to investigate what happens when Sp2 is deleted during neurogenesis at the embryonic and postnatal stages of forebrain development. She found that Sp2 is required for timely progression through mitosis within neurogenic niches, and the results of that work were published in Development in 2013. That is when Caroline joined the lab and the current paper is a summary of all the work she has done over that last few years. It is important to note that the use of MADM technology was instrumental in this work and our interactions with my friend and colleague Simon Hippenmeyer (a co-author on the 2013 paper) were critical in the initial stages of the project.
CJ My major focus was to dynamically investigate mitosis to understand how its timing was affected by loss of Sp2, as the previous project has identified a defect in M-phase progression. Additionally, I wanted to look at earlier stages of corticogenesis prior to the transition to neurogenesis when the stem cell pool is expanding. A very important finding that emerged from our work has been the revelation of phenotypic differences between bulk and sparse genetic deletion strategies. The complex genetic models we used revealed that bulk deletions can cause major non-autonomous effects that may mask the cell-autonomous role of a protein. By using sparse deletions, we are able to measure in detail genotype-phenotype associations.
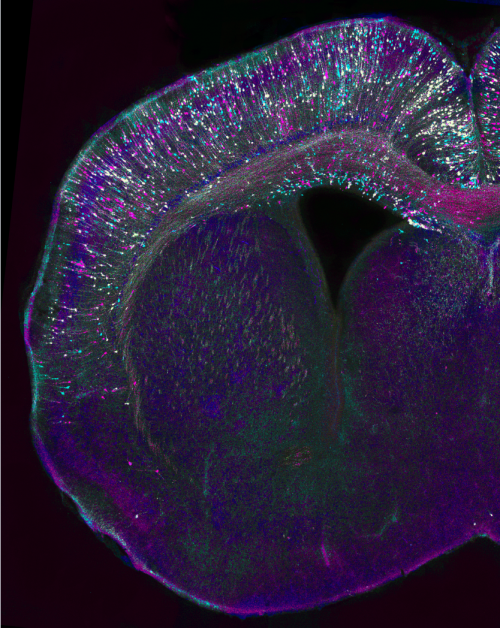
Can you give us the key results of the paper in a paragraph?
CJ Essentially, we found that Sp2 is required for neurogenesis during a distinct time point of cortical development. Surprisingly, Sp2 is dispensable for early stages of corticogenesis, when stem cells are amplifying and the switch to neurogenesis occurs, but later, when the production of upper layer neurons begins, loss of Sp2 affects the timing of mitosis and ultimately results in the decreased production of neurons.
Do you think that Sp2 acts primarily in neural progenitor cell or intermediate progenitor division when promoting the production of upper layer neurons?
CJ I think that ultimately disruption of mitosis in the neural progenitor population results in the decreased production of intermediate progenitor cells, but it is also possible that disrupted mitosis in the mother cell has consequences for the proliferative capacity of intermediate progenitor cells. An important next step would be to look at the timing of mitosis in the neural and intermediate progenitor populations at multiple neurogenic stages to see how their populations and mitotic timing are affected. Additional experiments specifically deleting Sp2 from the intermediate progenitor population would also be interesting.
TG While we did not distinguish between the two modes of terminal divisions in our study, we suspect both are affected. However, one has to do the experiment to answer the question directly. The results in our current paper reveal stronger effects on large clones and upper layer-specific sensitivity when Sp2 is deleted at sparse levels, which suggest both types of terminal divisions are likely affected in the absence of Sp2.
Do you have any idea about the transcriptional targets of Sp2 that might ensure timely mitosis?
CJ Recent studies of Sp2-dependent transcription have indicated it is involved in multiple pathways and may require different co-factors. It is unclear what the transcriptional activity of Sp2 is outside of cell lines and in developing tissues, whether or not it interacts with these potential co-factors within the developing cortex, and/or if it is playing a non-transcriptional role at one of these stages. Sp2 is known to interact with the nuclear matrix and may be regulating gene expression through a process independent of transcriptional regulation.
TG Our lab has been pursuing questions related to upstream regulation of Sp2 and downstream targets of this interesting protein, but more work is needed.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
CJ Realizing that larger clones had a stronger phenotype in response to loss of Sp2 was definitely exciting, as it indicated that the clones that were expanding throughout neurogenesis were most affected (similar to our results in the other experiments). The smaller clones, which likely reflected lineages that immediately entered neurogenesis, were not strongly affected.
Working with animal models is always more complicated than you would hope
And what about the flipside: any moments of frustration or despair?
CJ Of course, working with animal models is always more complicated than you would hope, and the early timepoint experiments were surprising at first because Sp2 has a known role in cell proliferation. However, doing the additional experiments with bulk deletion and an additional Cre driver line helped to reveal that we really weren’t seeing an effect at this early stage of cortical development.
So what next for you after this paper?
CJ I am working with Dr Ghashghaei on projects in the lab related to my previous project. In terms of the future, I’m interested in exploring scientific writing or clinical research careers to help advance biomedical research.
Where will this work take the Ghashghaei lab?
TG We are moving forward with a number of projects that continue to interrogate mechanisms that regulate the transitions that NSCs and NPCs in the forebrain go through as development proceeds.
Finally, let’s move outside the lab – what do you like to do in your spare time in Raleigh?
TG I have been playing soccer (football to the rest of the world outside America) for a long time. Playing with old friends as well as coaching and scouting for some of the local clubs forms much of my time outside the lab. In addition, my daughter is a gymnast in college – so I spend quite a bit of time with my wife going to her meets.
CJ Raleigh is a beautiful city and the veterinary school is located next to the art museum and greenway, so I like to run at the trails there. I also enjoy cooking and spending time at my parents’ farm.


 (No Ratings Yet)
(No Ratings Yet)