The people behind the papers – Chase Bryan and Kristen Kwan
Posted by the Node Interviews, on 30 September 2020
This interview, the 78th in our series, was published in Development earlier this year.
Optic cup development involves a series of intricate cell and tissue movements, and cells’ interaction with the extracellular matrix (ECM) is known to play an important role. However, the details of how ECM components work in eye development, and where they come from, is still poorly understood, and is the subject of a new Development paper that takes advantage of live imaging in zebrafish embryos. We caught up with first author Chase Bryan and his supervisor Kristen Kwan, Assistant Professor in the Department of Human Genetics at the University of Utah, Salt Lake City, to find out more about the story.

Kristen, can you give us your scientific biography and the questions your lab is trying to answer?
KK Thanks for asking! I am a cell and developmental biologist. I got my start as a biochemist studying membrane trafficking as an undergraduate in Suzanne Pfeffer’s lab at Stanford University. I worked with Marc Kirschner during my PhD, and it was during that time that I began thinking about morphogenesis; I worked toward understanding how developmental signals are integrated with the cytoskeleton and cell adhesion during Xenopus development. Marc gave me a lot of freedom to develop these ideas, and since then I’ve been fascinated by the problem. Knowing that I wanted to do live imaging, I went on to do a postdoc with Chi-Bin Chien, where I began working on eye morphogenesis in zebrafish. Chi-Bin was extremely supportive and helped me start to develop computational approaches to address this problem. My lab is currently working to understand the cellular and molecular mechanisms governing eye morphogenesis. When, where and how do cells move? How is the tissue organized? How does the embryo construct three-dimensional organs in a precise and stereotyped manner? We hope to answer questions like these by combining live imaging, computational methods, genetics and cell biology.
Chase, how did you come to work with Kristen and what drives your research today?
CB I met Kristen briefly prior to starting graduate school at the University of Utah – I was working as a lab technician at the time, and the postdoc I was working with asked if I wanted to go see his friend’s (Kristen’s) job interview seminar. When I saw the movies of zebrafish optic cup development she made during her postdoc and heard the pitch she had for her science (something like ‘you can understand so much of biology simply by watching it happen’), I knew I wanted to work with her and have her teach me those techniques. That same idea drives the work I am doing now as a postdoc.
What did we know about the ECM’s role in optic cup morphogenesis before your work?
CB & KK We know surprisingly little about the function of ECM molecules! Research over many decades has described the presence of ECM proteins around the developing optic cup in many different organisms, but much less has been discovered about ECM function. Work from other groups has demonstrated that the ECM protein fibronectin is important to establish the lens, as is laminin-1. Different subunits of laminin-1 have also been shown to regulate optic cup shape, cell polarity and retinal differentiation, but beyond these proteins not much is known about the functional role of other ECM components.
Can you give us the key results of the paper in a paragraph?
CB & KK Mesenchymal cells have been observed surrounding the developing eye for decades, and studies in mouse had demonstrated that mutants with disruptions to the periocular mesenchyme display optic cup morphology defects, but a specific role of either the mesodermal mesenchyme or the migratory neural crest have not been well established. In this research, we focused on the neural crest, as we had genetic tools ready at hand to try and parse out the role of those cells in optic cup morphogenesis. We found that neural crest mutants in zebrafish displayed optic cup defects, and observed that neural crest cells migrate around the developing eye throughout optic cup morphogenesis. We then found that neural crest helps establish the basement membrane that surrounds the retinal pigment epithelium. These neural crest cells express the ECM protein nidogen, and by disrupting nidogen function we found that this neural crest-derived nidogen is necessary for proper optic cup morphogenesis.
Why do you think optic vesicle cells move faster when the basement membrane is disrupted?
CB & KK We propose in our model that the basement membrane serves as a molecular handbrake for the movement of optic vesicle cells. The optic vesicle develops as a bilaminar epithelial tissue, so movement in one part of the epithelium could push or pull other parts of the tissue forward or backward, like a conveyor belt. The basement membrane could serve as an adhesive layer for the epithelia to stick to, and could thereby regulate the speed at which individual cells or the sheet as a whole get moved along. Without the basement membrane in place to adhere to, those cells could lose their footing, so to speak, and keep getting pushed or pulled along faster than they do in wild-type conditions.
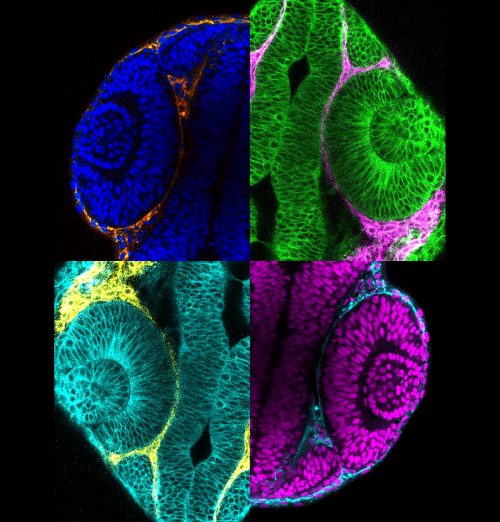
And do you think all of the defects you observe result from problems with cell movements, or might other mechanical or signalling aspects be affected?
CB & KK Other mechanical or signalling aspects could certainly be affected when neural crest or basement membrane assembly get disrupted. The ECM has many known roles in regulating movement and presentation of signalling molecules, only a couple of which we have directly tested. In terms of mechanical aspects, integrin signalling is one obvious molecular link between the ECM and the cells that adhere to it, which could affect the cytoskeleton and in turn regulate epithelial morphogenesis. There are also a lot of unexplored mechanical and biophysical aspects of morphogenesis, and the ECM may play into many other pathways, such as Hippo or tension receptors.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
CB I’m lucky because this project was full of satisfying little events. Some of the most memorable moments of my graduate work came from this project: taking a time-lapse image where the optic cup stayed in focus throughout the night and getting to watch the neural crest migrate around the eye, or looking at beautiful electron micrographs after spending an entire weekend prepping samples for electron microscopy. Perhaps the biggest moment like this came after I’d spent months engineering and raising the nidogen transgenics. To engineer the transgenes, inject the fish, raise multiple generations, and finally get to perform the heat-shock experiments and find that the transgenes worked just like I’d predicted was immensely gratifying.
“Some of the most memorable moments of my graduate work came from this project”
And what about the flipside: any moments of frustration or despair?
CB There was definitely some frustration working with a double mutant, and even more so when we started adding in transgenes. Even with zebrafish where you can have hundreds of embryos to work with at any given time, you can only work with a handful before they develop beyond the time point you’re trying to study. We had many experiments where Mendelian genetics worked against us and we didn’t get more than one or two embryos with the correct genotype.
So what next for you after this paper – I hear you’ve moved cross country?
CB That’s right, I recently started a postdoc with Marty Cohn at the University of Florida. I’m still keenly interested in the cell biology underlying morphogenesis – I’ve been developing a mouse organ culture system and am using the live-imaging techniques I learned from Kristen to study the formation of the mammalian urethra and to understand how normal development is altered in a condition called hypospadias.
Where will this work take the Kwan lab?
KK I am truly excited about where this work has led us: Chase’s work indicates that building the basement membrane is a collaborative endeavour between different tissues. Moving forward, we are extremely interested in identifying other ECM molecules provided by extraocular sources. Our preliminary data suggest that there are multiple cell types providing many different ECM molecules to the developing eye. We are excited to determine how these function in an integrated manner to support proper eye development.
Finally, let’s move outside the lab – what do you like to do in your spare time?
CB I enjoy figuring out how things work, and that expands beyond biology – before I was a cell biologist, I spent my free time rebuilding cars and motorcycles. I’m still an avid motorcyclist, but since moving to Florida I’ve also been taking advantage of the sun to get more into bicycling and triathlon training.


 (No Ratings Yet)
(No Ratings Yet)