The people behind the papers – Daniela Chavez and Gillian Stanfield
Posted by the Node Interviews, on 18 January 2019
This interview, the 55th in our series, was published in Development last year
Sperm development and differentiation are regulated by somatic cells and the extracellular signals they produce – often regulators of proteolysis. Premature or delayed differentiation can compromise fertility, and thus tight spatiotemporal control of the process is crucial. A paper in Development addresses how two secreted proteins control sperm maturation in the context of the C. elegans gonad. We caught up with the paper’s first author Daniela Chavez and her PhD advisor Gillian Stanfield, Associate Professor of Human Genetics at the University of Utah, to find out more.

Gillian, can you give us your scientific biography and the questions your lab is trying to answer?
GS Entering graduate school at MIT, I was interested in developmental biology: how can a single cell possibly transform into a complex organism? I was drawn to C. elegans for its genetics and simplicity, and the idea that one could analyse development at the level of individual, highly reproducible cell fate decisions and interactions, so I joined Bob Horvitz’s lab to work on the genetics of programmed cell death (apoptosis). Moving on to my postdoc, I became fascinated with germ cells, and now my lab uses the worm to study various aspects of sperm cell biology. We focus on two main areas – sperm differentiation and sperm competition – and how each of these processes is regulated to maximize reproductive success. In C. elegans, both sexes make sperm, but they use different genetic pathways to control their differentiation. Also, male sperm outcompete hermaphrodite sperm to fertilize oocytes, and we would like to understand the underlying cell biology that makes that possible.
And Daniela, how did you come to join the Stanfield lab, and what drives your research?
DC When I joined as a graduate student, I was already familiar with the research and with C. elegans because I had previously been an undergraduate student in the Stanfield lab. In fact, my graduate school lab rotations were all with other labs, but I returned for the opportunity to use genetics to study the cell biology of reproduction and for the rigorous training environment of the lab and department. My research is driven by my fascination with gamete cell biology and how their successes and failures can influence evolution and have big consequences for a species.
What was known about the roles of SWM-1 and TRY-5 in sperm activation before this paper?
DC & GS Previous work from the lab had shown that the serine protease inhibitor SWM-1 is required for male fertility. It ensures that sperm remain immotile within the male gonad. This is crucial because motile sperm are transferred inefficiently during mating. The TRY-5 protease had been shown to be a seminal fluid protein and sperm activation signal. While it was known that SWM-1 inhibits premature sperm activation via TRY-5, it was unclear which tissues expressed SWM-1 or where SWM-1 and TRY-5 might interact.
Can you give us the key results of the paper in a paragraph?
DC & GS We wanted to address the developmental question of where and when does SWM-1 function to inhibit premature sperm activation? We used integrated transgenes and a CRISPR-generated knock-in to determine SWM-1 protein localization and found that it is in seminal fluid: it surrounds sperm stored in the male and, unexpectedly, it is in body wall muscle. This led us to wonder which source of SWM-1 is important for inhibiting sperm activation, so we used tissue-specific expression in swm-1 mutants and found that the extragonadal source, body wall muscle, is the critical source of SWM-1. This was a surprising finding since body wall muscle is not a part of the gonad, and this result demonstrated that secreted factors outside the gonad can impact reproductive success. We also analysed SWM-1 and TRY-5 localization together and found that while TRY-5 is present at low levels near stored sperm, SWM-1 levels are higher. This suggested that, in the male, sperm are in an inhibitory environment, whereas in the uterus, after sperm are transferred, the balance is shifted toward activation. We were left wondering about the other source of SWM-1, in seminal fluid, and whether it has a role in the uterus. By overexpressing SWM-1 in seminal fluid, we found that high levels of SWM-1 in seminal fluid can reduce male fertility. While the role of SWM-1 in seminal fluid remains unclear, our experiment suggests that the level is regulated to achieve a balance that is optimized for sperm success.
What might be the benefits of producing such a crucial regulator of sperm activation in a distant tissue, rather than locally?
DC & GS It’s a great question. We think it may be beneficial to produce SWM-1 in a tissue that develops well before sperm are made to ensure that sufficient inhibitory signal is present as soon as meiosis is complete. After meiosis, sperm are poised to respond rapidly to activation cues. This is a critical feature because once they are transferred to the uterus, if they do not activate quickly they can be swept out of the uterus and lose the chance to fertilize an oocyte. We think that perhaps producing SWM-1 in a somatic tissue that develops prior to sexual maturity, rather than within the gonad, which develops coincident with sperm production, allows sperm to maintain their quick response characteristics without activating prematurely.
Do you have ideas of other roles for soma-to-germline (and germline-to-soma) signal exchange?
DC & GS Although there are other soma-germline signals described in C. elegans, we think there are likely many that are still unknown, given our and others’ findings that proteins can seemingly move freely from the body cavity to the germline. It will certainly be interesting to study the mechanism of exchange and to find other signals that can affect the germline this way.
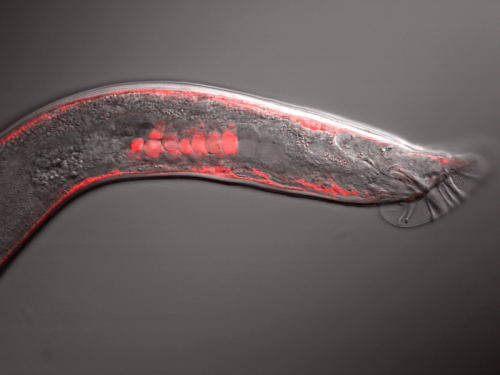
When doing the research, did you have any particular result or eureka moment that has stuck with you?
DC I will always remember the first time I saw SWM-1::mCherry in a worm under the microscope. It’s a pretty amazing feeling to see something that no one else has ever seen before. Fluorescent SWM-1 is especially mesmerizing to stare at since it is in several cell types and has distinct localization patterns in each tissue.
It’s a pretty amazing feeling to see something that no one else has ever seen before
And what about the flipside: any moments of frustration or despair?
DC Troubleshooting the CRISPR editing was a maddening process. It took about a year and a half trying different strategies that did not result in a knock-in. Looking back, I’m not sure why I kept trying and continued to have hope after so many failed attempts, but I am certainly glad I didn’t give up.
So what next for you after this paper?
DC I hope to study gamete biology for a long time to come. I am currently a postdoctoral fellow in the Department of Reproductive Sciences at the Smithsonian Conservation Biology Institute studying the other gamete: oocytes. My work focuses on understanding oocyte developmental competence and developing fertility preservation methods geared toward banking gametes of endangered species. Mixing my interests in gamete biology with conservation is a wonderful combination of my passions so I hope to find ways to continue on this path.
Where will this work take the Stanfield lab?
GS There are many interesting new directions to consider. In the short term, we are thinking about what Daniela’s results mean for our current projects. For example, using genetic screens, we have collected a set of new sperm activation mutants, and we would like to identify the corresponding genes. We had considered it likely that those would be expressed in the germ line or gonad, but now we realize that this set of candidates may be too limited, even as a first pass.
Finally, let’s move outside the lab – what do you like to do in your spare time in Utah and Washington?
DC I love to snowboard, camp and get a massage to recover from pipetting and microscope time. I also like to cook food inspired by my family’s culture, which includes Costa Rica, Mexico and Utah. On occasion, I sew mediocre Halloween costumes or amoeboid sperm-shaped bean bags. In DC I am enjoying commuting to the lab on a bike and trying to eat all the varied food the city has to offer.
GS Utah is a fantastic place to spend time in the outdoors. Living in Salt Lake, we are surrounded by mountains – I can leave my house and be skiing in an hour. A little farther away, southern Utah has spectacularly beautiful desert scenery, and I camp and hike there whenever I can. But currently I spend most of my spare time chasing my 6-year-old son!


 (1 votes)
(1 votes)