The people behind the papers – George Britton and Aryeh Warmflash
Posted by the Node Interviews, on 15 November 2019
This interview, the 71st in our series, was recently published in Development.
Our understanding of many fundamental aspects of early human development is still in its infancy, but a promising avenue for research uses advanced in vitro culturing techniques. For instance, confining human embryonic stem cells to micropatterned substrates and directing differentiation with signalling molecules has proved a powerful system to mimic (and readily perturb) events usually hidden in the embryo. A paper in Development now applies this technology to the question of how the embryonic ectoderm is patterned into defined domains of progenitor cells. We caught up with first author and graduate student George Britton and his supervisor Aryeh Warmflash, Assistant Professor in the Department of Biosciences at Rice University in Houston, Texas, to find out more about the paper.
Aryeh, can you give us your scientific biography and the questions your lab is trying to answer?
AW I was originally trained as a theoretical physicist and spent a good deal of time in graduate school developing techniques for modelling non-equilibrium systems, in Aaron Dinner’s lab at the University of Chicago. I also got involved in creating mathematical models of the development of the immune system and became convinced that the most interesting questions were in developmental biology. I realised that, although we had great collaborators for my graduate work, if I didn’t learn to do experiments, I would always be dependent on others to generate data. It really isn’t possible to make progress in biology from a purely theoretical standpoint. I was interested in working on symmetry breaking and spatial patterning, so, for my postdoc, I moved to Rockefeller University to work with Eric Siggia (a theoretician) and Ali Brivanlou, who studies early development using Xenopus frogs and human embryonic stem cells. I actually started out working with frogs, but found it to be a difficult system for quantitative imaging, and eventually switched to cell culture. During my time there, I developed the two main research directions which I have continued in my own lab – understanding the dynamics of morphogen signalling and how cells interpret them, and understanding how these signals are organised in space to generate patterns. In my lab, we are particularly interested in addressing these questions in early mammalian/human development. This is an attractive area because a lot is known about the identity of the signals that govern development during this time, and the phenotypes associated with their disruption, but much less is known about how these signals operate in space and time. I think that we know enough now to say that mammalian development works quite differently from other systems that have been dissected quantitatively – such as the Bicoid gradient in fly – and so it will be interesting to see what new mechanisms emerge. We have focused a lot of effort on understanding how the germ layers are patterned at gastrulation, and in our new study we extended these methods to a slightly later developmental time point – patterning one of those germ layers, the ectoderm.
George, how did you come to work in the Warmflash lab, and what drives your research today?
GB My previous research training involved the development of ultrasound-guided therapeutic nanoparticles functionalised to reduce necrotic and apoptotic cell death following a traumatic head injury, such as an ischemic stroke. Although our work led to significant improvements in neurological outcomes in animal models, the benefits were limited to early administration. Naturally, it was during this time that I became interested in harnessing the regenerative potential of neural progenitors to overcome the loss of functional tissue due to injury. At that time it was not clear to me what information a collection of transplanted neural progenitors needed and whether the same information could be used to repopulate neural function at any position in the brain. It was then serendipitous that I had the opportunity to join Aryeh’s lab at Rice University as a graduate student. He had developed a reductionist approach to understanding how human embryonic stem cells make fate-based decisions during gastrulation. I thought this was a powerful approach to untangling how a collection of spatiotemporally regulated signals give rise to particular arrangements of fate patterns. Although we aren’t regenerating functional brain tissue, I believe the approach and many of the principles learned can be applied to solve such problems in the future.
Before your study, how much was known about human ectodermal patterning and the extent to which studies in model organisms would translate to it?
GB & AW A great deal has been learned about how ectodermal patterning works in model organisms, but for the most part it wasn’t (and still isn’t) clear how much of this will translate to human. Most of what we knew about specifically human ectodermal development prior to the project was limited to directed differentiation protocols, which were formulated with knowledge gained from decades of experiments in model organisms. For example, the application of BMP and TGFβ inhibitors instructs the formation of neural progenitors from pluripotent stem cells, while appropriately timed BMP or WNT application can generate neural crest or placodes, respectively. So, much of the signalling appears to be conserved but there are also differences – for example, the order in which neural progenitors activate the key transcription factors SOX1 and PAX6 is different. One of the exciting things about micropatterned systems is the ability to compare species while standardising the geometry and culture conditions. However, this also requires the development of analogous systems with pluripotent cells from different species, which is a big job. This has begun to be done for gastrulation micropatterns: a system for mouse embryonic stem cells has been developed in Kat Hadjantonakis’s lab, and it would be interesting to do this for our system as well.
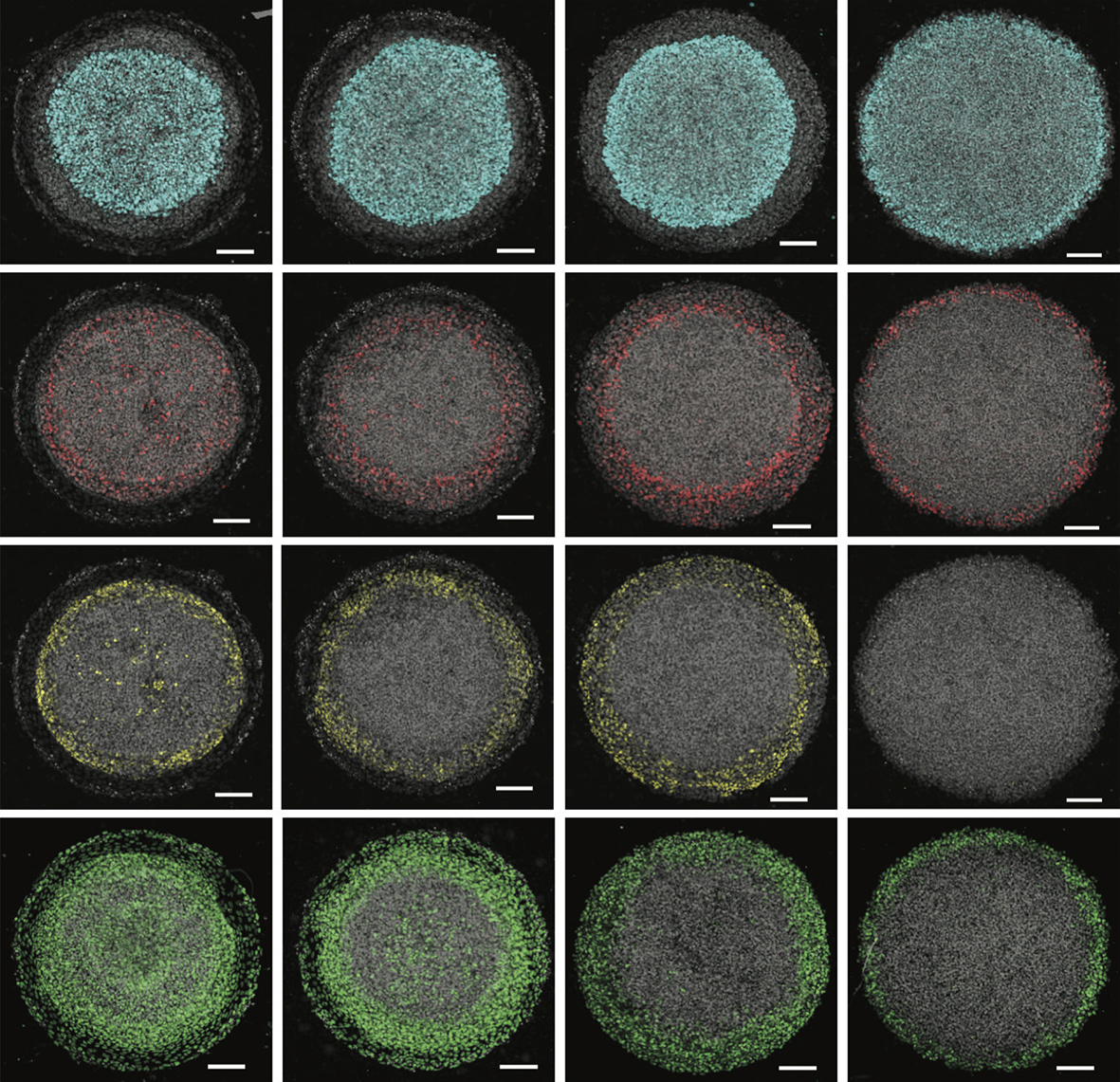
Can you give us the key results of the paper in a paragraph?
GB & AW We first mapped the competence of human embryonic stem cells to be diverted to alternative fates during ectodermal differentiation, and identified a time period when cells were primarily restricted to ectoderm but still capable of generating all ectodermal fates. We used this information to develop a platform that generates self-organised patterns of human neural, neural crest, placodal and epidermal progenitors on micropatterned surfaces with a similar organisation as found in vivo. While developing the protocol, we realised that inhibiting endogenous WNT signals was essential for limiting the extent of neural crest differentiation, which led us to identify WNT signalling duration as a crucial parameter controlling the decision between neural crest and placodes. We also showed that both BMP and WNT are involved in this decision, and that it is the relative rather than absolute levels of signalling that determines the patterns. Surprisingly, we also found that manipulating these signals during the initial ectodermal differentiation phase could influence their later competence. For example, early inhibition of BMP signals prevents cells from forming surface ectoderm in response to BMP later during patterning. Finally, we used the ease of manipulating the system to establish the necessity and sufficiency of these signals in activating particular key genes for neural plate border formation and patterning.
You find that the duration of WNT signalling can control ectodermal fates: how do you think this relates to what might be happening in the embryo?
GB & AW This is an interesting question and the answer is likely to be complex. At one level, the requirement to inhibit WNT signalling in our model probably arises because we only model one germ layer, not the interactions between germ layers. WNT inhibition in vivo has been shown to come from the visceral endoderm as well as the head mesoderm. Another role of WNT signalling is to set the coordinate along the anterior-posterior (AP) axis, and what we see is consistent with this – shortest WNT signalling yields anterior patterns with placodes and no neural crest, while the longest WNT signalling gives posterior patterns with neural crest and no placodes. Other studies within the nervous system and neural crest have shown that longer durations of WNT signalling are needed for more posterior fates. However, as WNT seems to induce neural crest specifically, it needs to do so at a particular position along the medial-lateral (ML) axis as well. So at each AP position, WNT signalling must also be controlled to give the correct ML pattern. How the AP and ML patterns are coordinated is an interesting open question.
How do you think cells measure the relative levels of WNT and BMP signalling at the molecular level?
GB & AW Another interesting question, and we are investigating two possibilities. It is possible that cells perform this calculation at the level of signalling through interactions between BMP and WNT, several of which have been described in the past. Alternatively, pathway interaction may not be required, and the computation could be performed by the downstream networks that govern cell fates. In the simplest example, if two mutually repressive transcription factors formed a toggle switch that implemented a binary cell fate decision, and one signal induced each of these transcription factors, this circuit would naturally take ratios between the transcription factor levels in making the cell fate decision. Of course, the real circuit is much more complex, but this serves to illustrate that transcriptional networks could definitely perform these computations on multiple inputs. To sort this out, we will need to measure the signalling activity through both pathways under different conditions as well as how different transcription factors interpret those levels.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
GB There was definitely one instance that I will remember for a long time. In the beginning of the project I had formulated a basic micropatterning protocol (the two-step induction protocol) that generated patterns of neural, neural crest and surface ectoderm. It was nice that patterns emerged at all, but I had grown frustrated with the lack of placodal expression. Thankfully, I had a patient advisor and a great platform to scan a range of signalling perturbations. Then, one late evening in the ‘scope room I observed a beautiful ring of SIX1 expression (a placodal marker) as a result of WNT inhibition. Thinking I was alone, I jumped and danced out of excitement but soon after noticed I wasn’t alone. In my moment of celebration, the janitor had stepped into the room for cleaning but found a grad student dancing alone with no music. It was a bit embarrassing at the time, but she had a great laugh.
I jumped and danced out of excitement but soon after noticed I wasn’t alone
And what about the flipside: any moments of frustration or despair?
GB I don’t think there is a single moment that stands out. It’s natural to run into roadblocks or become frustrated when developing a project from scratch. However, one point of frustration I learned to manage was the longer time required to conduct a single experiment compared with my lab mates, as most of them were studying gastrulation stage patterning with experiments that take 1-2 days. When it was time to present at lab meetings I consistently had fewer results to share, which led to feelings of self-doubt or insufficiency as a member in the lab. Eventually, with more experience, I developed strategies to increase the throughput of my experiments, which has helped push the project in directions I didn’t expect.
So what next for you after this paper?
GB Eventually I need to graduate, but I plan to conduct single cell analysis of signalling and fate in our ectoderm patterns as a follow-up paper. Unfortunately, I am still unsure what direction I should take for a postdoc but I am actively searching.
Where will this work take the Warmflash lab?
AW We are building on this system in a few different ways. We are quite interested in directly measuring the signalling dynamics of BMP and WNT with reporters that we have previously developed and used to study signalling during germ layer patterning. We are also interested in whether we can determine the AP position at which we are achieving this patterning and in developing protocols to control it. Finally, we are interested in extending this system to three dimensions to try to recapitulate some of the morphogenesis that the ectoderm undergoes during patterning, and to be able to look at how patterning and morphogenesis are coordinated.
Finally, let’s move outside the lab – what do you like to do in your spare time in Houston?
GB Unfortunately, most of my hobbies require a mountainous landscape. However, Houston makes up for its lack of interesting topology and good weather with an awesome food scene and a consistent line-up of touring indie bands. Tickets for live shows rarely get sold out and prices are student friendly, so it usually takes minimal advanced planning.
AW I have three kids aged 12, 9 and 5 so that keeps me quite busy outside the lab. We like travelling with the kids and doing things outdoors. It is very hot in the summer here, but the weather is nice the rest of the year and there are some great state parks not too far from Houston. We also like going to the Texas Hill Country and to the beach in Galveston.


 (No Ratings Yet)
(No Ratings Yet)