The people behind the papers: Holly Voges, Enzo Porrello & James Hudson
Posted by the Node Interviews, on 15 March 2017
The use of organoids – which can be defined as artificially grown masses of cells or tissue that resemble organs – in basic and clinical research has snowballed in recent years, providing insight into fundamental developmental processes and disease etiology. Today’s paper comes from the new Special Issue of Development devoted entirely to organoids, and reports the use of human cardiac organoids to address the regenerative capacity of the immature heart. We caught up with first author Holly Voges, and her co-supervisors Enzo Porrello and James Hudson, who jointly run the Cardiac Regeneration Lab in the University of Queensland.

So James and Enzo, how did you come to co-run the Cardiac Regeneration lab in the University of Queensland?
JH I graduated with a Bachelor of Engineering (Chemical & Biological) from the University of Queensland in 2006. Rather than getting a job in the mining/oil and gas industry I decided to do a PhD in tissue engineering as I found this a more rewarding career path for my inquisitive personality. I graduated with a PhD in 2011 under the guidance of Prof Justin Cooper-White who was one of the prominent Australian researchers at the forefront of bridging the engineering and biology disciplines. From here I really wanted to apply my skills and propel my research in cardiac tissue engineering to the next level. I therefore approached Prof Wolfram-Hubertus Zimmermann one of the world leaders in cardiac tissue engineering to do a postdoc in his lab. My work in Prof Zimmermann’s lab from 2011 to 2012 was in part supported by a postdoctoral research fellowship from the German Cardiology Society, which enabled me to develop new cardiac differentiation protocols and tissue engineering strategies as part of the team Prof Zimmermann had put together. I was then awarded a National Health and Medical Research Council of Australia Early Career Fellowship for 2013-2016 and therefore decided to move back to Australia to continue my research in cardiac tissue engineering. Upon returning to Australia my goal was now to build on my previous work and use human cardiac organoids (hCOs) to find novel therapeutics for patients with cardiac disease. This is when I teamed up with Dr Enzo Porrello who also had the same goal with his work in mice on cardiac regeneration. Ever since then we have been working together to decipher how cardiomyocytes regeneration is regulated in the hope of finding novel regenerative therapeutics for cardiac disease.
“We have been working together to decipher how cardiomyocytes regeneration is regulated in the hope of finding novel regenerative therapeutics for cardiac disease”
EP I completed my PhD at The University of Melbourne and Baker Heart and Diabetes Institute. My PhD thesis focused on the developmental origins of cardiac hypertrophy, which is when I first became interested in understanding the mechanisms controlling cardiomyocyte cell cycle arrest in the neonatal period. I was subsequently offered a postdoctoral position in Eric Olson’s lab at UT Southwestern Medical Center and I moved to Dallas in 2009. During my time in the Olson lab, working together with Hesham Sadek, we uncovered a previously unappreciated regenerative capacity of the neonatal mouse heart. My research since then has focused on understanding the mechanisms that control cardiac regenerative capacity during the neonatal period in mammals. When I returned to Australia in 2012 to set up my independent laboratory at The University of Queensland, one of the questions I was very interested in was whether the human heart retained a similar regenerative potential during foetal/neonatal life. At the time, pluripotent stem cell-derived cardiomyocytes were emerging as a new technology platform to study human cardiac biology. When I first met James Hudson in 2013 we discussed numerous aspects of cardiac developmental and regenerative biology for several hours and realised that we had a unique opportunity to combine our skill sets to address some important questions in the field. We merged our labs in 2014 and our joint research program takes advantage of our respective skill sets in human cardiac tissue engineering and in vivo mouse models to develop much needed regenerative therapies for heart disease.
What is Brisbane, and Australia in general, like for stem cell and regeneration research?
EP & JH There are world class researchers in Brisbane in this area, not only in cardiac but in different fields of research. Australia wide the community is also strong as the Australian government has supported a big stem cell and regeneration initiative “Stem Cells Australia” which started in 2011 ($21 million). The funding will run out soon so hopefully another program is funded to continue to move our research forward, as there has been some exciting progress. We also have an annual meeting run by “Australasian Stem Society for Stem Cell Research” which is attended each year by Australian and also prominent international researchers each year. The International Society for Stem Cell Research (ISSCR) annual meeting is the largest in our field, and next year it will be in Melbourne Australia so hopefully there will be a strong Australian contingent at this meeting next year.

And Holly, how did you come to join the lab?
HV I was lucky enough to join the lab as an undergraduate student when Enzo was setting up at UQ. I came across the lab profile online and thought his work was really interesting so I contacted him to see if I could do a 6 month project and here are we now, five years later!
Before you started your work, what was known about the regenerative capacity of human hearts of different ages?
EP, JH & HV Landmark papers from Enzo’s earlier work for the first time demonstrated that the regenerative capacity is lost during development in mammals. In contrast to the adult, newborn mice are capable of fully regenerating their hearts after injury (resection and myocardial infarction), a finding which is now widely supported by data from multiple labs. It has been known by clinicians for some time that in early life patients undergoing cardiac insults have better outcomes and heart recovery than adults. There has also been a recent case study demonstrating full recovery from myocardial infarction in a newborn. However, it was not fully understood whether this recovery/regeneration was due to cardiac regeneration or clinical management of these patients.
And what does organoid technology bring to the table for research into heart development and repair?
EP, JH & HV It provides us with an in vitro human model of cardiac regeneration we can use to complement our in vivo studies in mice. Mice and humans have many differences in their biological and physiological properties and models such as this give us an additional tool in our quest for regenerative therapeutics.
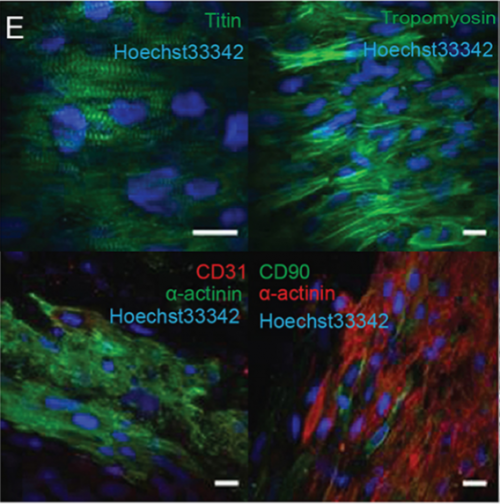
Can you give us the key results of the paper in a paragraph?
EP, JH & HV Our hCO display properties and maturity consistent with fetal-like human heart tissue. We developed an injury model in this paper to determine how the hCO would respond to injury. In this study we chose to model disease using cryoinjury because it enables us to injure part of the tissue whilst keeping the remainder of the tissue healthy and viable. If we used ischemia-reperfusion or a chemical insult (eg. cardiotoxin) it would have affected the entire tissue. We then showed that the cryoinjured tissues have a high cell cycle activity and they can regenerate and recover their functionality following injury.
Do you have any idea why regenerative capacity is lost with heart maturation? Would more ‘mature’ organoids be poor regenerators?
EP, JH & HV In our on-going work in the hCO and in vivo in the mouse we are starting to understand more about the maturation process and how this regulates cardiomyocyte cell cycle and regeneration. Part of our work is finding the key drivers of maturation in hCO and whether mature hCO have the capacity to regeneration. We believe that more mature hCO will lose their regenerative potential.
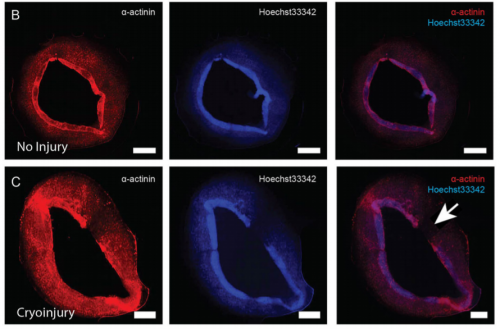
Will your model have relevance for clinical avenues to cardiac repair in adults?
EP, JH & HV Our hope is to use this model to find key regulators of cardiac regeneration (together with our in vivo mouse models). Through these studies, hopefully we can find factors that can be used to facilitate cardiac regeneration in adult hearts and patients with cardiac disease.
And Holly, when doing the research, did you have any particular result or eureka moment that has stuck with you?
HV There were a number of really exciting moments along the way and it is great to be surrounded by people who share this excitement. One memory that stands out was when I finished collecting the organ bath data. This proved to be a time-consuming and painful process, so when I finally saw the exciting results I immediately ran down the hallway to show James and Enzo.
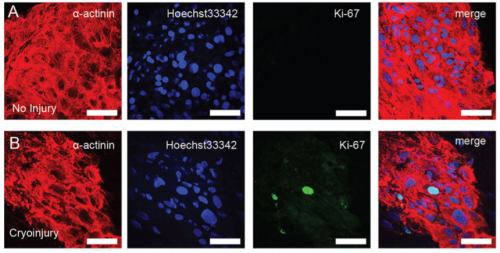
And what about the flipside: any moments of frustration or despair?
HV I definitely had many moments of frustration throughout this project. Most of the time it felt like the moments of despair outweighed the eureka moments, but that only made the eureka moments more exciting.
What about your plans for the future following this paper?
HV I’m currently working on my next paper, which will enhance our hCO system to be a step closer to resembling native heart tissue by incorporating other endogenous cell types.
And what next for the lab?
EP & JH We are working together and also with academic and industry collaborators to try and study the cardiac regeneration process and the molecular mechanisms governing cardiomyocyte cell cycle at different stages of development. The holy grail is to find novel regenerative therapeutics for cardiac disease.
Holly K. Voges, Richard J. Mills, David A. Elliott, Robert G. Parton, Enzo R. Porrello, James E. Hudson. 2017. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development, 144(6): 1118-1127
Browse the People behind the Papers archive here.


 (4 votes)
(4 votes)