The people behind the papers: Alaa Hachem & John Parrington
Posted by the Node Interviews, on 16 August 2017
Embryogenesis begins with fertilisation, and defective activation of the egg by the sperm is implicated in human infertility. Today’s paper, published in the most recent issue of Development, investigates the role of the sperm protein PLCζ in egg activation and the calcium oscillations that accompany it. We caught up with co-first author Alaa Hachem and his PI John Parrington, Associate Professor in Cellular and Molecular Pharmacology at the University of Oxford and Fellow of Worcester College, to hear more.

John, can you give us your scientific biography and the main questions your lab is trying to answer?
JP I studied at the University of Cambridge where I took Natural Sciences. An important inspiration for me was doing my third year studies at the Department of Zoology where scientists like John Gurdon, Ron Laskey, Michael Bate, and Alfonso Martinez-Arias, were harnessing the power of molecular biology to address some key questions in developmental biology. I then did my PhD at the Imperial Cancer Research Fund with Ian Kerr investigating how transcription factors regulate the activities of cells in the immune system.
My first post-doc saw a change of direction. I worked under the supervision of Karl Swann and Tony Lai at the National Institute of Medical Research trying to identify the molecular basis of the process whereby an egg is induced to develop into an embryo. These studies continued at University College London where I was able to continue my research into egg activation thanks to an MRC Career Development Award and an MRC Senior Research Fellowship. These studies culminated in the discovery – jointly with Karl Swann, Tony Lai, and Keith Jones – that the physiological agent of egg activation appears to be a novel sperm-specific protein called PLCζ. Subsequently, at the University of Oxford, where I moved to begin a lectureship at the Department of Pharmacology, my group were the first to show that mutations in PLCζ are associated with certain types of human infertility. PLCζ triggers egg activation by inducing Ca2+ signals in the egg.
Over the last decade, my group have been studying more generally the role of calcium signals in a variety of important pathophysiological processes
Over the last decade, my group have been studying more generally the role of calcium signals in a variety of important pathophysiological processes, in particular the role of the intracellular signalling molecule NAADP as a calcium mobilising messenger. These studies culminated in my discovery, in collaboration with Antony Galione, that two-pore channel, or TPC, proteins, are endolysosomal calcium channels regulated by NAADP. To study the mechanism of action and role of NAADP and TPC proteins, we generated knockout mice with loss of expression of TPC1 and/or TPC2. Our studies of these mice have identified important roles for TPCs in processes as diverse as smooth muscle and cardiac contraction, insulin secretion and sensing, autophagy and skeletal muscle function, intracellular trafficking, secretion of enzymes by the pancreas, brown adipose tissue thermogenesis, muscle development, and neo-angiogenesis.
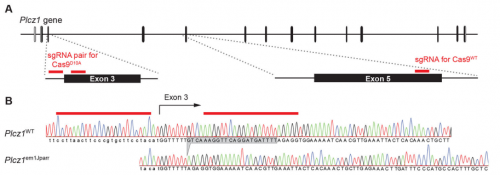
Most recently, I became interested in using the new CRISPR/Cas9 gene editing technology to create a PLCζ knockout mouse. The reason for this was that despite numerous studies pointing to an important role for PLCζ in the egg activation process, no one had provided the definitive evidence in the form of gene knockout that PLCζ was the physiological agent of egg activation. In fact, we had previously tried using the standard embryonic stem cell approach to make a PLCζ knockout but for some reason we never managed to get germline transmission. With only a limited budget for the project, when we heard about CRISPR/Cas9 gene editing, this seemed to offer a rapid and economic way to make a PLCζ knockout. And indeed we did, in a single generation, although from this we then generated two knockout lines, our studies of which are described in the Development paper.
As well as running a lab, you have been involved in public science communication and writing books on the genome and gene editing. How did you get in to this side of science, and how important is the public understanding of science to you?
JP I’ve been interested in the communication of science to the public ever since the start of my career as a PhD student. I’ve written about new scientific discoveries and the ethics and politics of science for various publications since that time and also taken part in a variety of science communication initiatives with young people. I have debated the science and ethics of cloning and assisted reproductive technologies with sixth form students, and even took part in a project with the Northern Ballet to teach cosmology to 14-15 year olds using dance and drama. For several years now, my wife Margarida Ruas – who is also a joint first author on the Development paper – and I have taken over the classroom of a year 6 (10-11 year old) primary school classroom, to give a lesson on ‘Sex, Reproduction, and DNA’. As well as a short Powerpoint presentation, this involves us showing the children fertilization of a sea urchin egg under the microscope, freezing objects in liquid nitrogen to mimic the freezing of human embryos in clinical ART labs, and the children extracting DNA from a strawberry. The popularity of this lesson was demonstrated by comments from the children such as ‘science is wicked!’ and ‘this is the best lesson I’ve ever had!’
You can learn more about the British Science Assication Media Fellows in this video.
To further my skills in science communication, I have also completed part-time diploma courses in science communication at Birkbeck College, London, and in creative writing at the University of Oxford. Although I’ve been involved in many science communication initiatives over the years, I think a crucial moment was being awarded a British Science Association Media Fellowship. This involved me working as a science journalist at The Times in London for six weeks in the summer of 2012. I really enjoyed covering all manner of different science topics during this period, and I was very pleased that 22 of my articles were published. This gave me confidence and helped develop my writing and communication skills.
One of the stories I published concerned ENCODE – the project whose aim is to map all the functional activity in the genome. This later became the subject of my first book – The Deeper Genome, published by Oxford University Press in 2015 – which tackles the controversial question of how much of the genome is junk and how much is important. My second book – Redesigning Life, published by OUP in 2016 – is about gene editing, but also other new approaches such as optogenetics and stem cell technology. I have just finished writing a book about genetics for secondary school students, and I’m working on one about the biology of human consciousness for OUP. So you could say I have caught the book-writing bug. It’s definitely a juggling act finding the time to write popular science books as well as doing my university teaching, research and writing papers, reviews, and grants. But I have really enjoyed being able to tackle broader scientific topics than I can in my research, and the extra readership that this has brought. It has also been fun to talk about the subjects covered by the books at science and literary festivals and have a debate with the audience, who can be of a variety of ages and backgrounds. All of which should make it clear that I take communication of science to the public, including the debate about its direction and ethics, very seriously.
Alaa – how did you come to join John’s lab? I understand your PhD is funded by the Iraqi government?
AH The first time I met John was during a fertility conference in Yazd, Iran, where he presented his latest studies of PLCζ’s role during fertilization and its link with human infertility. His talk caught my attention and I decided to approach him to ask him more about his future projects, and also about the chance of doing a DPhil in Oxford under his supervision. I found him very keen in extending my knowledge of the process of mammalian egg activation, and he was also very happy to help me with my application to Oxford. In 2013, I joined his lab after successfully securing a generous grant from the Higher Committee For Education Development in Iraq (HCED-Iraq). The prime minister’s initiative provided me with sufficient funds for covering the cost of college and university fees, accommodation and living expenses, as well as some money for the research itself.
Can you give us key results of the paper in a paragraph?
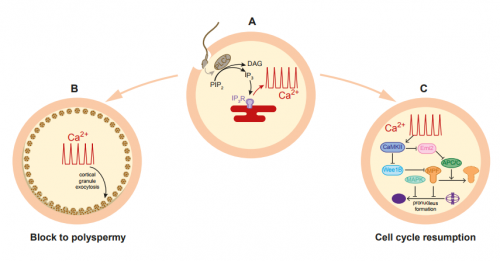
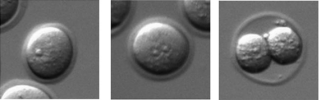
AH&JP Activation of the egg by the sperm is the first, vital stage of embryogenesis. The sperm protein PLCζ has been proposed as the physiological agent that triggers the Ca2+ oscillations that normally initiate embryogenesis. However, there has been no evidence that gene knockout of PLCζ abolishes the ability of sperm to induce Ca2+ oscillations in eggs. We used CRISPR/Cas9 gene editing to generate PLCζ knockout mice. Our studies of sperm from PLCζ knockout males showed that these fail to trigger Ca2+ oscillations in eggs. This therefore provides the first definitive evidence that PLCζ is the physiological trigger of these Ca2+ oscillations. Remarkably, however, some eggs fertilized by PLCζ-null sperm can develop, albeit at greatly reduced efficiency, and after a significant time-delay. In addition, PLCζ knockout males are subfertile but not sterile, suggesting that in PLCζ’s absence, eventually egg activation can occur via an alternative, although much less efficient route. This is the first demonstration that in vivo fertilization without the normal physiological trigger of egg activation can result in offspring.
In the absence of Ca2+ oscillations, how do you think egg activation occurs?
AH&JP The unfertilized egg is kept in a state of meiotic arrest by the maturation promotion factor, or MPF. A later decline in MAPK activity correlates with the formation of pronuclei and entry into interphase of the first cell cycle. Previous studies have shown that MPF levels can be reduced artificially in various ways, even without a Ca2+ stimulus, triggering egg activation and development to blastocyst in vitro. This mode of egg activation, without Ca2+ release, may explain the spontaneous activation observed by previous studies in ovulated, hamster and mouse eggs left to reside in the oviduct for extended periods of time in the absence of fertilization. Moreover, eggs from the C57BL/6 strain, the strain used in our studies, have been shown to have a particularly high susceptibility to spontaneous activation during in vitro maturation. Therefore a related mechanism might be responsible for the activation we observe, with the self-activation that aging, unfertilized eggs experience that normally results in fragmentation or embryonic arrest, being rescued by the PLCζ knockout sperm. A more intriguing possibility is that some stimulus supplied by the sperm, either a soluble factor, or the product of sperm-egg binding, is triggering egg activation in the absence of PLCζ. Although we failed to observe the Ca2+ oscillations typical of fertilization following ICSI or IVF with PLCζ knockout sperm, it remains possible that PLCζ knockout sperm are inducing an atypical Ca2+ signal in the egg that somehow we failed to detect in the current study. Examining this possibility will be an important focus for future studies.
How might your PLCζ mice be useful for studying male infertility and its treatment?
AH&JP Currently infertility in men whose sperm fail to induce egg activation, can be treated by inducing egg activation artificially with mechanical, electrical, or chemical stimuli. However, the long term effects of such treatments on human development remain far from clear, an issue of some concern given that the Ca2+ signals induced by these treatments are highly non-physiological. The availability of PLCζ knockout sperm now makes it possible to assess in a mouse model, how artificial egg activation stimuli, used in the clinic, might affect embryonic gene expression and offspring growth, metabolism and behaviour. Importantly, it will provide a way to test the efficacy and safety of recombinant PLCζ protein as an alternative therapeutic agent to treat infertility caused by egg activation deficiency.
When doing the research, did you have any particular result or eureka moment that has stuck with you? And what about the flipside: any moments of frustration or despair?
AH Eureka moments are always a joy to share with friends and colleagues. However, such moments don’t come easily as they are usually preceded by great amounts of frustration and despair. I remember the moment when we observed the genotyping results of the mice produced after performing the CRISPR-Cas9 gene targeting of the PLCζ gene. The results showed us for the first time that mutation by out-of-frame deletion had occurred, and all the guide-RNAs had worked successfully with both wildtype Cas9 and Cas9 nickase. That was a big moment of relief because previously we tried to mutate the coding sequence of the PLCζgene in mouse zygotes, however we weren’t successful due to a one nucleotide difference between the guide-RNA we had designed based on the standard mouse genome sequence and the genomic DNA of the strain of mice used for the gene targeting. At the time, I felt extremely unlucky for this rare mismatch to have occurred, as it ruined five months of my work. However, a friend told me that it doesn’t show how unlucky I am, but rather how ‘special’ I am for this rare event to occur during my scientific career.
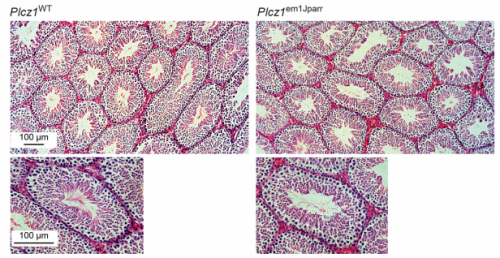
Another moment that really pleased me was when I collected the epididymis from the first homozygous knockout male at the age of 8 weeks. A previous unpublished conference report had claimed that knockout of the PLCζ gene was detrimental to the spermatogenic process, leading to no sperm being produced, which would have made it impossible to use gene knockout to test the physiological importance of PLCζ in the egg activation process. Thankfully, these claims didn’t hold true as I discovered that male PLCζ knockout mice were able to produce sperm with normal parameters of viability, motility, and capacity to undergo capacitation and the acrosome reaction. This allowed us to confirm PLCζ as the source of the Ca2+ oscillations at fertilization which otherwise would not have been possible.
The PLCζ knockout mice allowed us to begin unravelling long-awaited questions regarding the identity of the ‘sperm factor’ that triggers embryogenesis and its role in the egg activation process. Initially, I assumed that the males we had created would be totally infertile, given that their sperm lacked the capacity to trigger the normal signal that induces embryogenesis. Yet, one day we received an email from our co-author Jonathan Godwin saying that some of the mutant males had managed to impregnate a few of the wildtype females. This was puzzling, and I initially assumed it was due to a mistake in labelling the homozygous mutants. However, the genotyping results of the new offspring confirmed they were heterozygous animals and that the knockout males had not been mislabelled. Therefore, in order to address this unexpected finding, we cultured zygotes generated with KO sperm by in vitro fertilisation and in vivo mating, which confirmed occurrence of activation in mice eggs in the absence of the normal Ca2+ oscillations, albeit with greatly reduced efficiency and after a significant delay. Importantly, the findings showed for the first time birth of individuals of a mammalian species in the absence of the normal physiological stimulus, and we have also witnessed the importance of PLCζ for achieving the normal physiological levels of fertility in mammals.
What are your career plans following this work?
AH I am an aspiring academic and so inherently I am drawn to teaching and research. Therefore, I plan to go back to Iraq where I hope to teach university students in embryology along with setting up my own lab where I will continue to study aspects of mammalian reproduction and infertility. My goal is to help develop the scientific community in Iraq. I believe that my studies at the University of Oxford has equipped me with the knowledge and skills to have a positive impact on both students and research. In the future, I hope to translate the findings clinically by working with infertility clinics in Iraq.
My goal is to help develop the scientific community in Iraq
And what next for the Parrington lab?
JP Providing the definitive evidence by gene knockout that PLCζ is the physiological trigger of the Ca2+ oscillations that initiate embryogenesis has been very exciting for me, since I have been on the search for the identity of the ‘sperm factor’ that kick-starts life ever since my first post-doctoral post. However, our findings raise as many questions as they answer, and I’m particularly keen now to study how egg activation and even development to term can occur in the absence of the physiological agent of egg activation. Given that some previous studies have shown that embryo development is sensitive to differences in the pattern of Ca2+ signals in the egg, there is also the interesting question of whether the offspring conceived by PLCζ knockout males differ in important respects, e.g. in their growth, metabolism, behaviour, lifespan, etc. compared to offspring conceived in the normal fashion. This issue also has clinical importance given that artificial egg activation stimuli are now being used to treat certain types of human infertility in which egg activation fails to take place without assistance. Given that such stimuli generate a Ca2+ signal that is quite different from the normal sperm-induced Ca2+ oscillations, we would like to use the PLCζ knockout sperm as a null background to test the efficacy and safety of these clinically used artificial egg activation stimuli in an animal model. In addition to these PLCζ-focused studies that I would like to carry out in the future, I am also very interested to continue my studies into the mechanism of action and pathophysiological role of the TPC endolysosomal Ca2+ channels that we are also investigating using gene knockout approaches.
Finally, what do you like to do when you are not in the lab?
AH I usually enjoy watching sci-fi movies and some sport activities when I have time.
JP As well as writing popular science articles and books, I read a lot of popular science. I also read books about history, politics, and a fair number of novels. I also like to travel and I usually try and learn a bit of the language of the country I’m visiting. I’m a political activist, and I’m particularly interested in the ethics and politics of science. For relaxation I like walking, running, exercising at the gym, cooking, listening to music, and watching the occasional film. Otherwise, my two children keep me pretty busy!
Hachem, A., Godwin, J., Ruas, M., Lee, H. C., Ferrer Buitrago, M., Ardestani, G., Bassett, A., Fox, S., Navarrete, F., de Sutter, P., Heindryckx, B., Fissore, R.
and Parrington, J. 2017. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 144:2914-2924.
This is #26 of our People Behind the Papers series; the full archive can be viewed here




 (2 votes)
(2 votes)