The people behind the papers – Li-Juan Duan and Guo-Hua Fong
Posted by the Node Interviews, on 23 May 2019
This interview, the 62nd in our series, was recently published in Development
Vascular development critically involves pruning, which helps to remodel an immature network containing excess microvessels into a mature and functioning one. The mechanisms of vascular remodelling and the relationship between the endothelial cells and the other cell types with which they are closely associated are, however, currently poorly understood. A new Development paper now demonstrates a crucial role for oxygen sensing by astrocytes in vascular remodelling of the mouse retina. We caught up with the two-author team behind the paper: research associate Li-Juan Duan and her supervisor Guo-Hua Fong, Professor of Cell Biology at the University of Connecticut Health Center, to hear more about the story.
Guo-Hua, can you give us your scientific biography and the questions your lab is trying to answer?
GHF After graduating from Hangzhou University in 1982, I attended the PhD Program in Biochemistry at the University of Illinois at Urbana-Champaign. My interest in angiogenesis began during my postdoctoral training with Martin Breitman and Janet Rossant in Toronto. After reporting the finding that Flt-1 (VEGFR1) was a negative regulator of vasculogenesis, I set up my first lab at the University of Western Ontario before relocating to where I am now (UConn School of Medicine).
Early in my career as a developmental biologist, I used mouse embryos to study vasculogenesis and angiogenesis, but since about a decade ago I have been primarily focused on mouse retinas. At the molecular level, my current focus is on oxygen sensing and hypoxia signalling because growth of blood vessels is mostly (although not exclusively) an adaptive response to tissue hypoxia. Molecules of interest include hypoxia inducible factor (HIF) 2α (also called EPAS1), a transcription factor stabilized by hypoxia that activates the expression of angiogenic factors, and prolyl hydroxylase domain (PHD) proteins (EGLNs), which sense oxygen and destabilize HIFα proteins by oxygen-dependent prolyl hydroxylation reactions.
Li-Juan: how did you come to join the Fong lab and what drives your research?
LJD With a few exceptions, almost all tissues are vascularized. Mechanisms ensuring that blood vessels are formed at the right place, right time and with right configurations are very fascinating to me. Dr Fong’s lab investigates the molecular and cellular mechanisms regulating blood vessel growth, and has contributed significantly to this rapidly advancing field. So the opportunity to be a part of team and contribute to the exciting discoveries is a major driving force for my research. Also, the potential that knowledge generated from these studies might improve angiogenesis therapies is quite energizing.
What was known about the link between astrocytes, endothelial cells and oxygen in retinal angiogenesis prior to your work?
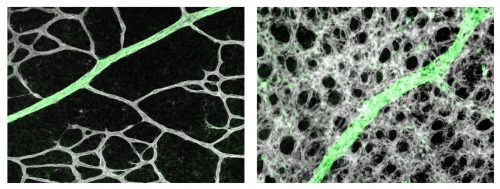
LJD & GHF The retinal astrocytic network is situated just beneath the vascular network on the inner retinal surface, with foot processes from astrocytes wrapping around vascular endothelial cells (ECs) and supporting their survival. It was thought that the astrocytic network forms before retinal angiogenesis and serves as a template for the subsequent vascular growth. The newly formed vascular network exhibits a honeycomb pattern consisting of almost uniformly sized capillaries, separated by roundish non-vascular tissues. To gain circulatory functions, the nascent vascular plexus undergoes active remodelling to form a tree-like pattern with large trunks and smaller branches. During this process, excess microvessels are pruned away. One widely held but never formally documented view is that oxygen causes pruning by activating EC apoptosis. The other theory is that T cells interact with ECs to cause pruning. In both theories, astrocytes were not suspected to play a role in remodelling.
Can you give us the key results of the paper in a paragraph?
LJD & GHF We found that retinal astrocytes respond to changes in tissue oxygenation mostly through prolyl hydroxylase domain (PHD) protein 2, an enzyme known to use O2 and oxoglutarate as substrates to hydroxylate transcription factors HIF1α and HIF2α, causing them to degrade. In neonatal mouse retinas, HIF2α is essential for the expansion of the astrocyte population owing to its role in maintaining astrocytes in immature and proliferative states. Angiogenesis imposes a physiological limit to the abundance of retinal astrocytes by causing O2-dependent HIF2α degradation. As such, a proportion of the numerous capillaries generated through robust angiogenesis is unable to acquire astrocytic support and undergoes regression (pruning). In support of this theory, targeted disruption of the Phd2 gene in astrocytes led to accumulation of HIF-2α protein, expansion of retinal astrocyte population, and persistence of extra microvessels. When astrocyte growth was directly stimulated by intraocular injection of PDGFA into the eyes in wild-type mice, vascular pruning was also blocked. Based on these findings, we conclude that oxygen- and PHD2-dependent astrocyte growth arrest is a feedback mechanism to prevent runaway vascularization.
Do you have any ideas about what happens downstream of Phd2/HIFα to induce differentiation in astrocytes?
LJD & GHF We don’t really know yet, but there may be some candidates. HIF2α is known to promote the expression of Oct4, a transcription factor important for the maintenance of stem cell identity. While the astrocyte precursors may not be exactly stem cells, they might share certain features related to stem cells. So it seems to us that HIF2α-dependent expression of Oct4 might be a potential mechanism by which HIF2α helps maintain astrocytes at precursor and immature states. At this point, this idea remains highly speculative but it could be a reasonable starting point for the next step.
Does your work have any relevance to pathological contexts?
LJD & GHF Capillary dropout is a common feature in early stage diabetic retinopathy. The prevailing view at present is that capillary dropout starts with apoptosis of pericytes, a type of supporting cell that adheres to the endothelial cells. Our finding of a crucial role for astrocytes in capillary maintenance raises the question of whether the adverse effects of diabetes on retinal astrocytes might also contribute to capillary dropout? If so, the retinal astrocytes could be a novel therapeutic target for improving retinal vascular stability. In this regard, it may be interesting to note that there’s a publication indicating that retinal astrocyte abundance is decreased in diabetic rats (Ly et al., 2011).
Our findings are also related to retinopathy of prematurity (ROP), a disease associated with premature birth. Whereas pathological mechanisms underlying ROP are complicated and go beyond oxygen alone, sensitivity to oxygen is nonetheless an important component. Thus, targeting astrocytic PHD2 could provide a novel opportunity for minimizing the consequence of ROP.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
LJD I guess the moment was when I found that simply injecting PDGFA into the eyes caused both expanded astrocyte populations and persistence of more microvessels, whereas injection of PBS into the contralateral eyes didn’t. These results indicated that increased astrocyte abundance alone was sufficient to block vascular pruning.
And what about the flipside: any moments of frustration or despair?
LJD Sure, plenty of them. This was actually a lengthy project. While I’ve been working on other projects in parallel, this project was on and off for nearly 6 years. While we observed the overcrowded vascularization at the very start of the project, it was rather challenging trying to explain why. Naturally, our initial attention was on VEGF, and lots of time was wasted in that direction. We couldn’t just publish overcrowded capillaries without being able to explain the underlying mechanism, so the project was really stuck for a long time until one day we realized perhaps it was simply the number of astrocytes itself rather than any specific molecule. We also ran into a lot of technical difficulties. One example was growing primary retinal astrocytes: because they could not be expanded too much, we had to pool the cells from a very large number of retinas. These are just two of the many examples.
Where will this work take the Fong lab?
GHF We will be pursuing several directions. We are interested in identifying the potential targets downstream of PHD2/HIF2α by RNA-seq, and then assessing their functionality in retinal vascular development by CRISPR/Cas9-mediated knockout. We will be also looking into potential interaction between HIF2α with a transcription factor called Nr2e1 or Tlx. We are interested in finding out whether the two transcription factors collaborate in retinal astrocytes in controlling astrocyte differentiation. We are also interested in investigating if PHD2 deficiency in astrocytes also confers protection to capillaries in disease models such as oxygen-induced retinopathy and diabetic retinopathy.
For whatever reasons, the vascular biology community has paid very little attention to astrocytes. The major focus is still on endothelial cells, and their interaction with pericytes, vascular smooth muscle cells and leukocytes. In fact, we also stumbled into this subject accidentally, but we increasingly appreciated the opportunity that fell upon us as we began to realize the importance of this cell type to vascular growth, stability and function. Because so little is known about astrocytes in the context of vascular biology, there are plenty of exciting findings to be made. In the coming years, we will be devoting much of our efforts trying to understand how astrocytes communicate with endothelial cells to regulate various aspects of vascular homeostasis.
Because so little is known about astrocytes in the context of vascular biology, there are plenty of exciting findings to be made
Finally, let’s move outside the lab – what do you like to do in your spare time in Connecticut?
LJD Spring is beautiful in Connecticut. I enjoy growing flowers, watching them blossom in the morning. There are also plenty of water reservoirs with hiking trails open to the public. I enjoy taking long walks around the lakes on weekends, especially in the spring and fall.
GHF Connecticut may not be a frequently talked-about place but it is not exactly in the middle of nowhere either – in fact the UConn School of Medicine campus is just about 2 h from Boston and New York, respectively. So it’s relatively convenient to spend a day in the weekend visiting museums or friends there, or even shopping. Also Connecticut is full of very long hiking trails at different levels of difficulty, so one could spend hours (or days if they like) hiking to fairly distant locations, with some leading all the way into neighbouring states.


 (No Ratings Yet)
(No Ratings Yet)