The people behind the papers – Diane Shakes & friends
Posted by the Node Interviews, on 20 September 2017
Development often involves the asymmetric partitioning of cellular components to daughters, and this process is crucial for successful gametogenesis. Today’s paper, published in the current issue of Development, explores the cytoskeletal mechanisms of spermatogenesis in different nematode species. We met the multi-lab team behind the work, starting with Diane Shakes (The College of William and Mary in Williamsburg, VA), and then her collaborators André Pires-daSilva (University of Warwick, UK), Gunar Fabig and Thomas
Diane, can you give us your scientific biography and the main questions your lab is interested in?

DS I got my start in research as a high school senior through a special program at NASA-Ames Research Center.There I was paired with a fantastic mentor, Patricia Buckendahl, who has talent for productively incorporating novice young scientists into her research quest which at the time was to understand the fundamentals of bone metabolism and why astronauts were losing bone mass in zero gravity. As an undergraduate at Pomona College, NASA-Ames continued to be my summer research home as I explored the breadth of biology in my coursework. Ultimately, I was captured by the wonders of cell biology and the big questions of developmental biology. It was also during this time that I developed an appreciation of the special insights that can be obtained by studying unusual organisms and cell types.
As a Ph.D. student at Johns Hopkins, I joined the research group of Sam Ward. This choice not only linked me not only to the early community of C. elegans researchers but also immersed me in the exciting research that was going on at the Carnegie Institution, Department of Embryology. In Sam’s lab, I worked alongside postdoctoral fellow Steve L’Hernault to isolate and phenotypically characterize a large collection of spermatogenesis-defective mutants in C. elegans. Through these studies, I developed an appreciation for genetics, a love for microscopy, and a life-long interest in the mechanisms of cell polarity. As I neared the end of my graduate studies, Ken Kemphues had just published his foundational study on the C. elegans PAR mutants, so I was excited to join his lab for my post-doctoral studies. My project was to analyze par-5, which like the other par proteins is required to establish proper asymmetries in the 1-cell C. elegans embryo, and ultimately was found to encode 14-3-3.
When I subsequently established my own lab, first at the University of Houston and subsequently at the College of William and Mary, I decided to use my combined knowledge of C. elegans sperm and oocytes to investigate a pair of C. elegans mutants that had been reported to exhibit both maternal and paternal effect defects.

Within my own lab, Penny Sadler discovered that although affected oocytes and sperm were both arresting in metaphase of meiosis I, the sperm continued to develop post-meiotically into anucleate sperm that could nevertheless crawl and fertilize oocytes.And in a fruitful collaboration with Andy Golden and a generous supply of mutants from the Bowerman and Seydoux labs, we showed that these and other mutants with the same phenotype were temperature-sensitive alleles of the anaphase-promoting complex. These studies that came out this work stimulated my interest in the interplay between the various cellular and developmental sub-programs of gamete development and drew me back into the analysis of C. elegans spermatogenesis, particularly in the stages leading up to meiotic divisions. The next set of studies – an analysis of the spermatogenesis-specific events during meiotic prophase – were carried out in collaboration with Diana Chu whose expertise in chromatin complemented my own in the cell cycle and cytoskeleton.
In addition to on-going studies in C. elegans, my group has also started using what we know about gametogenesis in C. elegans as a basis for comparative studies in other nematodes. A phone-call from André Pires da Silva got us specifically interested in the trioecious (male/female/hermaphrodite) species Rhabditis sp. SB347 (now called Auanema rhodensis), a lab cultivable nematode with strikingly non-Mendelian sex ratios. In many ways, these cross-species comparisons are analogous to studying a very informative mutant; but in this case, they help us distinguish highly conserved, fundamental processes from those that have been subject to variation over evolutionary time.
What was known about the cytoskeletal drivers of sperm development and asymmetrical positioning in worms before your study?
DS A conserved feature of sperm development in all organisms is that, following the meiotic divisions, sperm become streamlined by discarding unnecessary cellular components. In the early 1980s, Sam Ward’s group had shown, that in C. elegans, these unnecessary components included both actin and microtubules. This is only possible because nematode sperm motility is driven by a completely different cytoskeletal protein, the major sperm protein (MSP). Subsequent experiments with actin and microtubule inhibitors suggested that actin was more important than microtubules in this process of asymmetric partitioning. Subsequently, the L’Hernault and Titus labs showed that proper partitioning required the non-conventional myosin (myosin VI). Yet, no one had ever gone back to study the stepwise progression of events that underlies this wholesale swap of the cytoskeletal system during sperm development in C. elegans. Were there aspects of the process that could be better understood in light of new studies of asymmetric partitioning? Was the unusual partitioning event in R. sp. SB347 completely novel, or an informative variant of events that happen in all nematode sperm?
André – how did your collaboration with Diane come about, and why are different nematode species such useful models for the evolution of sex and reproduction?

APS Sex determination is a developmental switch prone to rapid evolution, but the causes and consequences for this pattern of evolution are poorly known. The existence of species with three sexes caught my attention because they are supposed to be extremely rare. In 2004 Marie-Anne Félix published a paper mentioning the free-living nematode strain SB347 (now named Auanema rhodensis), which produces males, females and hermaphrodites. Until that time, other free-living nematode species producing three sexes were not available in culture, or were parasitic nematodes difficult to work in the laboratory. Back in 2009, I contacted Diane Shakes to help me in characterizing the cytology of SB347 spermatogenesis, because we were trying to understand why males of this species generate so few males. This was especially puzzling, since heterogametic XO males should produce XX and XO progeny in equal proportions. However, we observed mostly XX progeny only. I contacted Diane because of her expertise in cell biology of C. elegans spermatogenesis and her recent interest in comparative work.
Gunar – I understand that an interest in C elegans sperm mutants brought you, Anna and Thomas into the collaboration?

GF As a PhD student, I am working in the lab of Thomas’ on chromosome segregation in C. elegans male meiosis. Our lab has a strong expertise in live-cell imaging and electron microscopy. So, some years ago I started to image living C. elegans males by fluorescence microscopy to analyze the dynamics of meiotic chromosome segregation. We also started to characterize wild-type spindles of various stages at the ultrastructural level using electron tomography. During the initial phase of my project, I started to think about a comparison of wild-type and mutant data to study situations of impaired chromosome segregation. At the time, I was aware that Diane had a very interesting paper together with André, in which they characterized male meiotic spindles in Rhabditis sp. SB347 (now A. rhodensis). In this paper, they reported about a skewed sex ratio that was most likely caused by a changed meiotic “program” during male chromosome segregation. So we contacted Diane and André to ask whether they would be interested in collaborating with us on the ultrastructure of male meiotic spindles in Rhabditis sp. SB347.

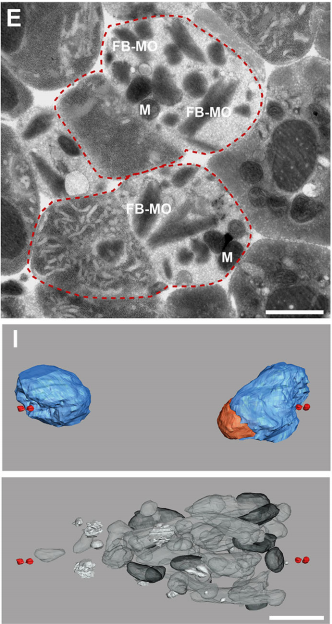
TMR At the same time Anna started in my lab to work on her Master’s thesis and I proposed to her that an EM analysis of males of this species would be a very exciting project. She did a terrific job in preparing and analyzing SB347 males. Anna discovered the interesting patterns of organelles partitioning to the respective daughter cells.
Jessica – how did you get recruited, and how did your previous work on non-centrosomal microtubules fit into the story?

JF One of the interests of my lab is to understand the mechanisms underlying non-centrosomal microtubule organization. C. elegans is a particularly good model in which to study this question as microtubule organizing center (MTOC) activity is completely reassigned from the centrosome in dividing cells to another site following mitotic exit. I had been exploring this switch in MTOC activity in a number of different cell types in C. elegans and started to focus on the germline, where MTOC activity is at the plasma membrane of non-dividing germ cells, and at the centrosome of mitotically dividing germ cells or meiotically dividing spermatocytes. I started to film this transition and found that microtubules and microtubule minus end proteins remarkably appeared to move from the centrosome of dividing spermatocytes to the residual body. This behavior appeared to mimic a similar movement of microtubules and microtubule regulators that I had previously seen in embryonic intestinal epithelial cells during their polarization, but due to the geometry of the movement in spermatocytes was much easier to visualize there. I presented this work at a conference that Diane was also attending. I think my live imaging data helped shed light on some of the observations Diane had been making about microtubules in fixed samples. In an incredibly gracious act, Diane contacted me to see if I would like to incorporate my data into this manuscript.
Can you give us the key results of the paper in a paragraph?
DS We knew from our earlier paper that the meiotic divisions of R. sp. SB347 male spermatocytes yielded a 50:50 mix of functional X-bearing sperm and residual bodies containing the other chromosomal complement. However we didn’t understand much about the details of the process or whether this pattern was an oddity of a single species. In fact, no one had described the stepwise process by which C. elegans sperm partition into residual bodies, not only unneeded cellular organelles but also their entire pool of actin and microtubules. In this study, we address all of these questions using the combined approaches of immunocytology, transmission electron microscopy, and live-imaging of GFP constructs. We show the first time that in C. elegans, the microtubules redistribute with their gamma-tubulin ring complexes intact from the centrosome to the sperm-residual body boundary in a process that resembles microtubule shifts in differentiating cells. At the same time, in a variation of normal cell division, actin reorganizes through a combination of cortical ring expansion and clearance from the poles. Relative to these cytoskeletal changes, organelles appear to partition in at least two phases; most partition just after the completion of anaphase chromosome segregation while others partition as the sperm detach from the residual body. In the much smaller spermatocytes of both R. sp. SB347 and its near relatives, the cytoskeletal remodeling events are restricted to the pole of the X-bearing chromosome set. Consequently, a partitioning process that is normally bipolar with two haploid sperm generating a central residual body becomes unipolar and generates one functional sperm and one DNA-containing residual body. Intriguing, this unipolar partitioning process also occurs in the XX spermatocytes of SB347 hermaphrodites. In contrast, partitioning is bipolar in the large spermatocytes of R. sp. SB347’s closest known male/female relative. Taken together, this study reveals two major insights. First, the process by which nematode sperm discard actin and tubulin into their residual bodies may be variations of common cellular and developmental processes. Second, constraints related to spermatocyte downsizing may have contributed to the evolution of a sperm cell equivalents of female polar bodies.
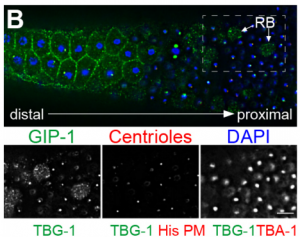
What did the TEM bring to the story?
GF & TM Spermatocytes had to be visualized in whole worms, so we had to perform serial sectioning for this project. Anna mastered this without any difficulties. The beauty about electron microscopy is that one can get very detailed images of cellular organization (e.g. about centrioles, microtubules or the Golgi apparatus). For electron tomography, we prepared whole males of Rhabditis sp. SB347 by applying high-pressure freezing. Males were ultrarapidly frozen to liquid nitrogen temperature, freeze-substituted and embedded them in Epoxy resin. We took overview images of serial sections of several worms and counted the number of different organelles in numerous cells as worms contain many spermatocytes. This information enabled us to quantify the distribution of organelles during various stages of meiotic cell divisions in males.
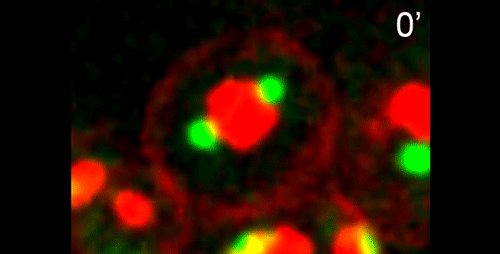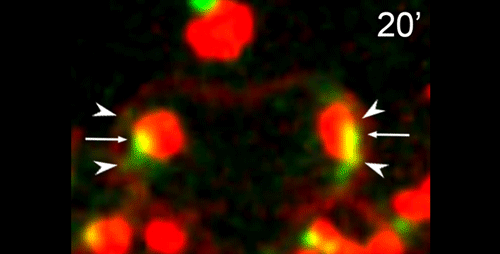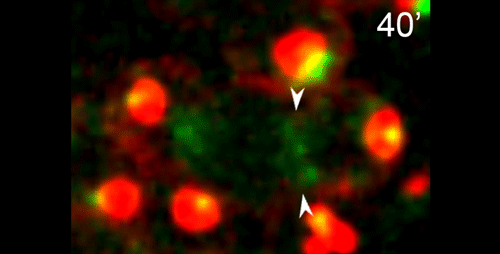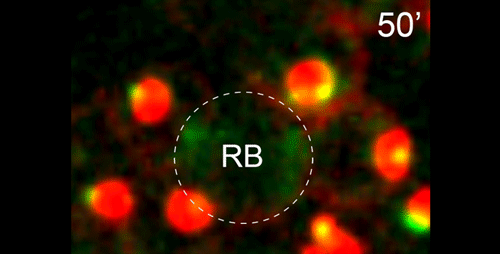
What are the key open questions about the role of microtubules in the asymmetric cell division that makes nematode spermatocytes?
JF To me the most interesting questions about the microtubules in nematode spermatocytes are, 1) how is the centrosome inactivated as an MTOC?, 2) how are microtubules transported to the RB?, 3) what organizes microtubules inside the RB?, and 4) in Rhabditis sp. SB347, what controls the selective inactivation of MTOC activity at only one of the centrosomes? The answers to these questions will not only teach us about spermatogenesis, but will also answer major questions in the field of microtubule organization.
What do you think the main evolutionary implications of the work are?
APS Three-sexed species are interesting, because they are probably evolutionary transitions between male/female and male/hermaphrodite mating system as known in C. elegans. They may help us understand in how mating systems evolve, a long-standing question in Evolutionary Biology. In this paper, we showed the mechanisms of ‘how’ males generate non-functional nullo-X sperm. This is one of the few examples in the literature describing cellular mechanisms of how heterogametic animals generate progeny of a single karyotype. There are examples for other nematodes and animals in other phyla in which crosses between XX and XO individuals generate mostly only XX progeny. Perhaps they use similar mechanisms as SB347, and this paper provides the foundation for testing this hypothesis. At the moment, however, we do not understand ‘why’ SB347 males get rid of their ‘male-making sperm’. According to evolutionary theory, this would happen if sibling matings are common. Unfortunately, we do not know much about the ecology of SB347 to test this.
DS From my perspective as a cell and developmental biologist, I think that this work highlights how a conserved set of cellular and developmental sub-programs can be co-opted for different outcomes. This study suggests that highly unusual process of partitioning microtubules into a residual body is not a novelty of nematode spermatogenesis but rather a variation of a common process during which differentiating cells lose their centrosomal microtubules as they establish a distinct population of non-centrosomal microtubules. Similarly, we show that the step-wise progression by which nematode sperm partition actin into the residual body exhibits many similarities to actin remodeling during the transition from anaphase to cytokinesis. Thus I predict that much of the underlying molecular machinery will be evolutionarily conserved with the notable exception of a novel regulator or altered feedback loop. Yet because these processes within nematode sperm are indeed a bit extreme, their analysis will yield important insights into related cellular and developmental processes.
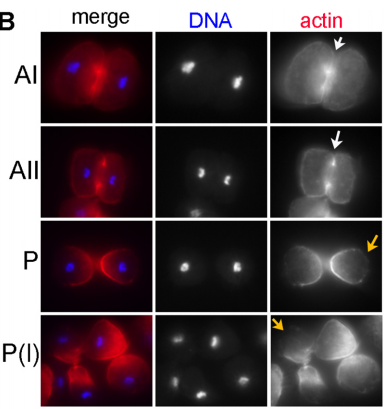
What next for the Shakes lab?
DS Our on-going analysis of R. sp. SB347 continues to surprise and delight us. There is still much to be learned about the molecular mechanisms of this partitioning event as well as other oddities of SB347 meiosis. For example, we also have a paper coming out this month in Developmental Biology, in which we describe how this clade evolved a distinct solution for acquiring hermaphrodite self-fertility, namely the simultaneous rather than sequential production of oocytes and sperm. So in the R. sp. SB347 realm, we have many interesting avenues to explore. At the same time, the C. elegans focused cohort in my lab are hard at work gleaning new insights from the existing collection of spermatogenesis mutants, some of which have been languishing in our liquid nitrogen tanks since my graduate days.
Any plans for future collaborations?
Yes, Andre and Diane’s lab are collaborating on a project that describes differences in meiosis in SB347 males, females and hermaphrodites, and the labs of Andre, Diane and Thomas are collaborating on a project to identify the signal that determines the directionality of the asymmetric division of the SB347 male spermatocytes.
Anything else you would like to add?

DS One voice that is missing in this interview is the lead author Ethan Winter, a former undergraduate honors student in my lab who helped spearhead this project through his cytological studies of R. sp. SB347 and its near relatives. Ethan continued on to Harvard to pursue a Ph.D. in Chemical Biology. Not only was he brilliant, hard-working, and tenacious, but he was also kind, patient, and possessed a wonderful quirky sense of humor. We all predicted that he was on track to develop into a beloved professor. Sadly, Ethan passed away last October (2016). I hope that this paper serves at least in a small way as a tribute to his scientific accomplishments and his too-short life.
, , , , , , ,
This is #27 in our interview series. Browse the archive here.


 (2 votes)
(2 votes)