A domino effect on brain developmental evolution
Posted by jorgetorrespaz, on 6 December 2019
The discipline “Evo-devo” studies the developmental basis of morphological evolution. In the field, some original animal models are emerging as interesting model organisms, enriching the knowledge in the field more and more.
In the DECA team (Développement et évolution du cerveau antérieur, in French) we use an Evo-devo approach to study the developmental mechanisms responsible for brain evolution. Adaptation to new environments brings along changes in brain morphology and function, and these changes are more striking in organisms adapted to extreme environments. A beautiful example that illustrates this topic is the model species used in our lab, the Mexican fish Astyanax mexicanus (teleost). Within the same species there are several river-dwelling populations (surface fish) and other populations adapted to caves (cavefish), living in absolute darkness. Evolution in the caves have led to the total loss of eyes and pigmentation, similarly to many other cave organisms. Since the cavefish embryos initially develop a small eye primordium that then degenerates at larval stages, Astyanax mexicanus has become an attractive model to study the genetic and developmental causes of eyes loss and the mechanisms of sensory compensation.

In our lab some hypotheses have been tested in order to explain the regression of the eyes in cavefish, including modifications of midline signaling centers important for eye induction, or “trade-offs” within the neural plate between the eye field and other neural tissues.
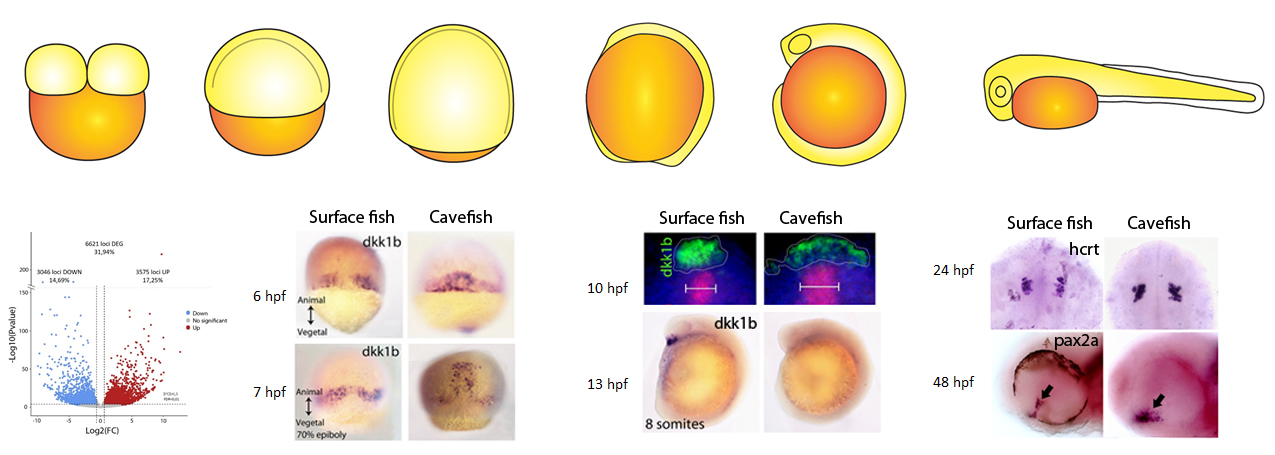
Recently we started to wonder how early in embryogenesis we were able to find differences between the two eco-morphotypes. We decided to compare the process of gastrulation in the two Astyanax morphs, to look at the establishment of the axial signaling centers important for brain development. By comparing systematically the expression of key genes involved in gastrulation we found important heterochronic differences in terms of internalization and migration of the precursors of axial mesoderm, the embryonic organizer. At this point we were very proud of ourselves, because we were able to discriminate between surface fish and cavefish embryos already at the onset of gastrulation, just by looking at in situ hybridizations.
We realized that at the end of gastrulation the anterior axial mesoderm, also called prechordal plate, was different in several aspects, particularly when we looked at the expression of dkk1b (an inhibitor of the WNT pathway). In cavefish embryos, expression of dkk1b occurs in cells that were more dispersed than in surface fish, with lower levels of transcripts and with an earlier off-set of expression, suggesting a globally reduced repression of WNT compared to the surface morphotype. In vertebrates, WNT repression is fundamental for the normal development of the forebrain, including the eyes. Through functional test we showed that modified WNT modulation is indeed implicated in the cavefish eye phenotype.
Next, we reasoned that if at the onset of gastrulation there is already a morphotype-specific embryonic patterning, there might be something different even before this step. Since embryogenesis before gastrulation, including the induction of the organizer, is controlled by determinants present in the oocyte before fertilization occurs, we made the hypothesis that differences from gastrulation onwards are could be due to modified synthesis of maternal determinants. Thus we decided to compare through RNAseq the transcriptomes in embryos just after fertilization, when only maternally-provided mRNAs are present (maternal transcriptomes). When we got the first results we were quite surprised: more than 30% of the maternally-expressed genes were differentially expressed in the two morphs, and around 6600 different loci were de-regulated!
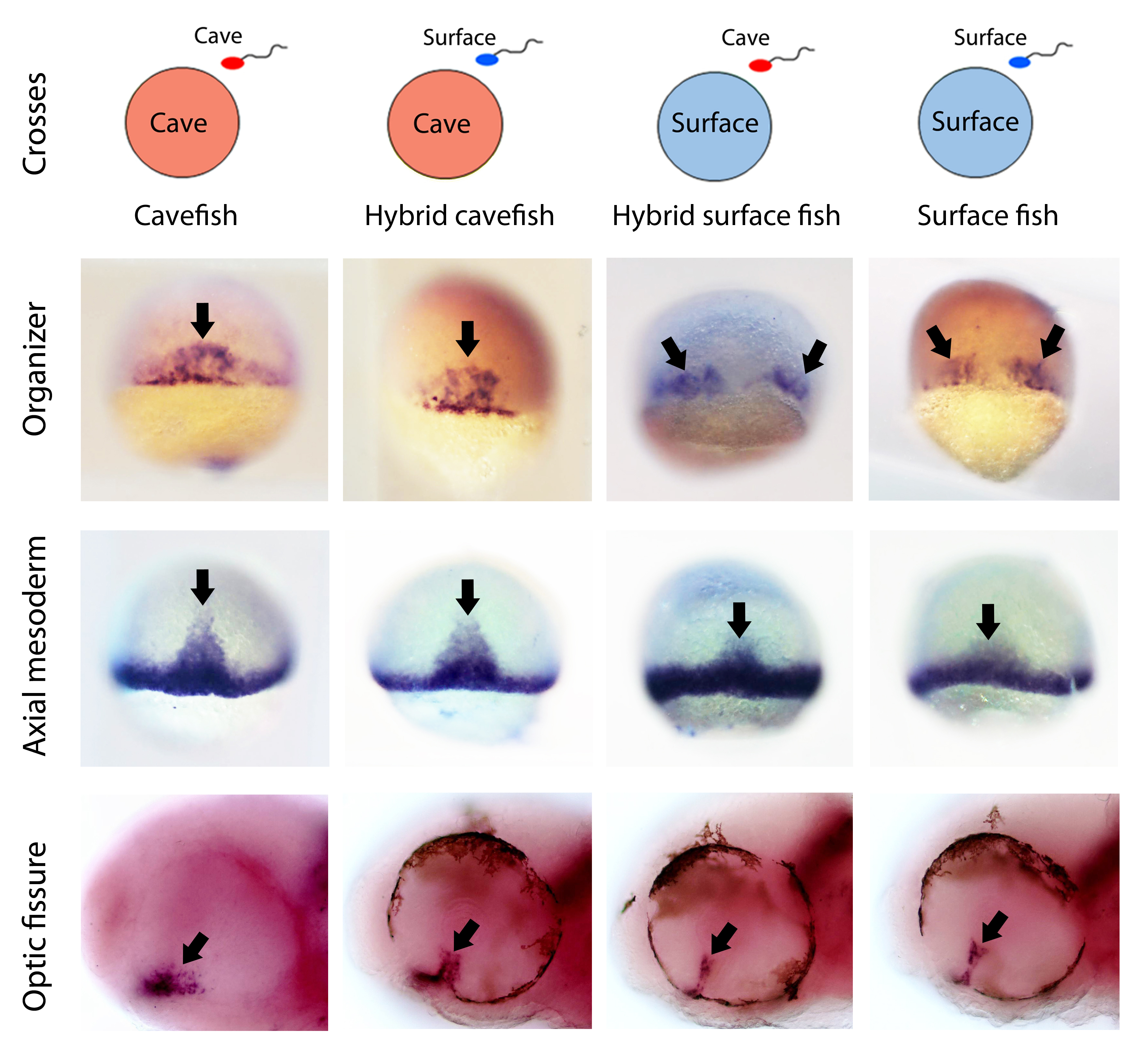
Then of course we wanted to test the maternal contribution to the cavefish phenotypic evolution. One of the greatest advantages of Astyanax mexicanus as a model is the interfertility between the different populations. In fact, in nature there are some geographic spots where hybridization of cave and river populations occurs nowadays. This unique feature allowed us to test the maternal contribution to a given phenotype by comparing F1 hybrids obtained by reciprocal crosses (hybrids obtained from surface fish eggs versus hybrids obtained from cavefish eggs). If the phenotype under study in the hybrids resembles the phenotype of the female morphotype, then we can say that there is a maternal effect on that trait. The results obtained here were also striking. We found that the patterns of gene expression in hybrids up to the end of gastrulation were exactly the same as those of their maternal morph. These results show first, that the morphotype-specific pattern of gastrulation is determined by maternal factors, and second, that maternal determinants influence embryonic development until stages much later than the maternal-to-zygotic transition.
After gastrulation, as development continues, all phenotypes analyzed became progressively similar between the reciprocal hybrids, and intermediate between the two pure eco-morphotypes. However, some of these morphotype-specific phenotypes at larval stages (hypothalamic patterning and eye regionalization) showed clear differences in the reciprocal hybrids, with a tendency towards the maternal morphs. This indicates that, when the zygotic genome (in a hybrid condition) takes over the control of development, it tends to homogenize the phenotypes, making less clear the full maternal effect observed at earlier stages.
Our work provided clear evidence that changes in the composition of the oocytes can modify the developmental trajectories, thereby contributing to phenotypic evolution. In this sense, the maternal transcriptome could react under specific environmental conditions, modifying subsequent interdependent developmental events, leading finally to a particular phenotypic outcome, in a way similar to a domino effect.
This work offers a new field for us to dig in. There are some questions we started to ask ourselves, for example could maternal genes be under selective pressure? Which mechanisms could account for their regulation within the ovary? How different is the maternal-to-zygotic transition in Astyanax morphs?
Research done by Jorge Torres-Paz, Julien Leclercq and Sylvie Rétaux at the Paris-Saclay Institute of Neuroscience, CNRS UMR9197, Université Paris-Sud, Université Paris-Saclay, France.
Reference:
- Torres-Paz*, J. Leclercq* and S. Rétaux. 2019. Maternally regulated gastrulation as a source of variation contributing to cavefish forebrain evolution. elife 8: e50160. doi: 10.7554/eLife.50160 (* Equal contribution).


 (6 votes)
(6 votes)