The people behind the papers – Charles Sheehan, John McMahon and Debby Silver
Posted by the Node Interviews, on 27 May 2020
This interview, the 76th in our series, was published in Development earlier this year.
Interneurons are crucial to cortical function and their dysregulation has been implicated in various neurological pathologies, yet how they are generated during development is still poorly understood. A new paper in Development investigates interneuron neurogenesis in the mouse embryo and its control by Magoh, a component of the exon junction complex. We heard more about the work from the paper’s two first authors, Charles Sheehan and John McMahon, and their supervisor Debby Silver, Associate Professor at Duke University Medical Center in Durham, North Carolina.

Debby, can you give us your scientific biography and the questions your lab is trying to answer?
DS I am broadly trained as a developmental neurobiologist. I have had the opportunity to pursue undergraduate research on the biochemistry of the cytoskeleton, PhD studies on cell migration in Drosophila, and postdoctoral research on neural development of the cerebral cortex and neural crest in mice. I started my independent lab at Duke Medical Center in 2010, where I am now an Associate Professor. The overall goal of my lab is to elucidate fundamental principles governing brain development and contributing to neurodevelopmental pathologies. We specifically investigate development of the embryonic cerebral cortex. A main focus is to understand post-transcriptional control, a fascinating but poorly understood regulatory layer of brain development. We have several questions of interest, including how RNA-binding proteins control cortical development at a cellular and molecular level, and the role of subcellular RNA localization and local translation in neural progenitors. We are also fascinated to understand the genetic underpinnings of human cortical evolution. We are guided by the premise that the same mechanisms at play during normal development were co-opted during evolution and, when dysregulated, can cause neurodevelopmental disease.
John and Charles, how did you come to work with Debby and what drives your research today?
JM I have always been driven to apply science to gain a better understanding of disorders, with the goal of ultimately developing novel therapies to treat a clinical need. After finishing my graduate studies, I came across Debby’s lab, which was doing fascinating work and working on very translatable science.
CS I first learned about Dr Silver’s work when I was applying for Research Technician jobs in 2017. When I interviewed with her, there was a genuine shared excitement for science that convinced me to join her lab.
What first got you interested in the role of post-transcriptional regulation in interneuron development?
JM Interneurons have been of interest to me since my graduate work on epilepsy. In my opinion they have been a largely understudied and underappreciated cell type, especially given their prominent role in numerous disorders. Likewise, there are significant gaps in our understanding of post-transcriptional regulation in both ‘normal’ and disease states. Therefore, I found exploring the role of the exon junction complex in interneuron development a particularly exciting project to work on.
DS Development of cortical inhibitory neurons is essential for establishing brain circuitry and is relevant for the etiology of many neurological disorders. Yet in contrast to the field of excitatory neurogenesis, and despite a lot of excellent work being done, I have been struck by the fundamental gaps in our understanding of the genesis of interneurons. For example, we lack a basic understanding of the role of the cell cycle in directing interneuron fates, as well as how RNA regulatory modules shape development. Given our lab’s prior work on Magoh’s role in the cortex, I felt we were in a unique position to make contributions to this field and address these knowledge gaps. I was also motivated to pursue this line of research given the increasing human genetic data linking the exon junction complex to neurodevelopmental pathologies.
Can you give us the key results of the paper in a paragraph?
JM The findings of the paper center around the integral role of Magoh in interneuron progenitors. We find absence of Magoh is detrimental to the balanced production of progeny in neural stem cells. We identify that a contributing factor to the pathogenic brain development is the activation of the p53 pathway. These findings raise important questions about the role of post-transcriptional regulation in neurogenesis.
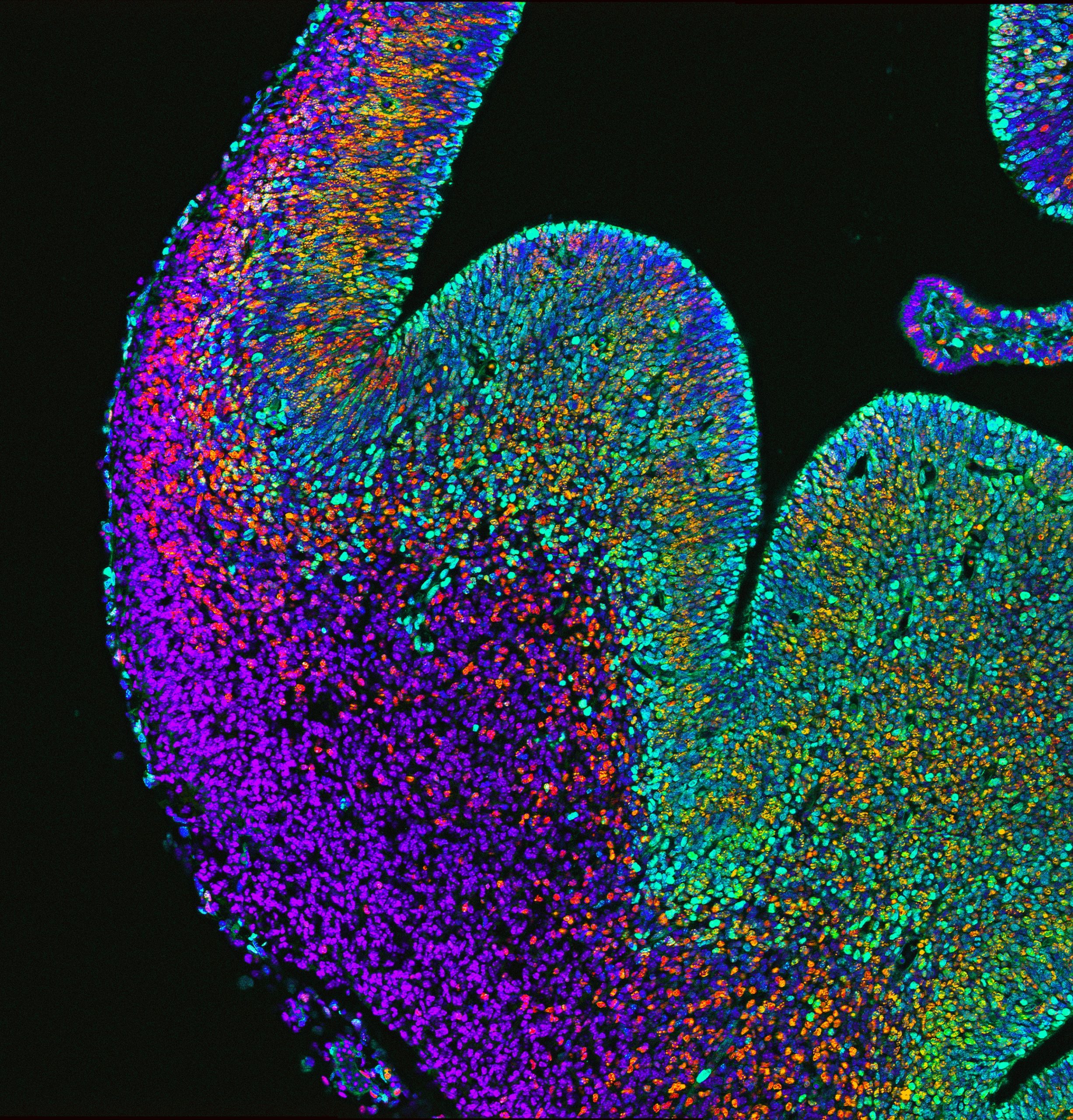
What do you think explains the difference in the effect of p53 loss in Magoh heterozygotes compared with homozygotes?
JM I think it really comes down to a dose-dependent function of Magoh, and likely the exon junction complex. We have seen dose dependence in a number of our conditional mutants, which points toward a criticality in the balance of EJC components. If a cell loses some Magoh expression, it seems to drive a less severe phenotype. A more extensive depletion results in a striking amount of cell death. My suspicion is that there is a fundamental role of the EJC components in mitosis, which we have yet to fully uncover.
CS We believe that p53 loss differentially affects Magoh heterozygotes and homozygotes because of the severity of their mitotic defects. Simply put, p53 deletion cannot rescue daughter cell death if the progenitors can’t divide. How Magoh dosage causes these differential phenotypes via transcriptome alteration and the effect that p53 loss on has on these changes would be of interest to understand.
DS What we discovered in this study, as well as in prior research, is that cells undergoing mitosis are exquisitely sensitive to Magoh levels. Losing a single copy of this gene causes progenitors to be delayed in mitosis, resulting in inappropriate activation of p53 signaling in newborn progeny, likely due to accumulating DNA damage. We postulate that in Magoh homozygotes, interneuron loss is caused by two defects: p53-dependent apoptosis of newborn cells and p53-independent depletion/death of precursors.
What does your study suggest about the role of EJC components in neurodevelopmental pathologies?
JM This study, along with others from the Silver lab, highlight that EJC components are absolutely necessary for proper function of neural stem cells, likely serving a critical role in mitosis.
CS This work suggests that neurodevelopmental pathologies caused by EJC mutations may not be caused solely by dysfunction of excitatory progenitors, but also interneuron progenitors. This finding gives a more complete understanding on how mutations in Magoh, and other EJC components, alter neural stem cell function and cause neurodevelopmental disorders.
DS Copy number variations and point mutations in EJC components are linked to intellectual disability, autism and microcephaly. Our study, together with prior work from our lab, suggests that mutations in EJC components disrupt proper generation and survival of both excitatory and inhibitory neurons. It may be that in patients carrying EJC mutations, there is imbalanced production and/or survival of these two populations or there may be qualitative aberrant defects in viable interneurons. Historically, microcephaly is linked to abnormal mitosis, with a heavy emphasis on cortical neurogenesis. Our study indicates that mutations affecting mitosis of interneuron progenitors may also be relevant for the etiology of microcephaly.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
JM After a session of live imaging, reviewing the videos always provided me with tremendous insight into the dynamics of neural progenitors and cell divisions. Not necessarily a eureka moment, but very insightful and absolutely beautiful to watch.
CS I think the most memorable eureka moment was when I was imaging the p53 rescue brains. Immediately when looking at the cKO, you could tell that there was still a drastic loss of cortical interneurons, which was unexpected based on what we knew at the time.
Many who have been at the bench for a while will tell you science is not for the weak hearted
And what about the flipside: any moments of frustration or despair?
JM I think many who have been at the bench for a while will tell you science is not for the weak hearted. From what I have seen, even the best scientists suffer more failures than successes. There were plenty of late-night experiments that were revealed to be pure failures once the results were in, and plenty of time spent trouble-shooting technical issues before seeing the light at the end of the tunnel.
CS Similar to the eureka moment, the p53 KO rescue experiments were quite challenging. The mouse genetics required to obtain the samples was a difficult and long process. Even once we had the data, interpreting how p53 was functioning took several discussions and looking back to other data until we reached on our current model.
So what next for you two after this paper?
JM Since my time in Debby’s lab I have been working in regulatory affairs, and furthering my understanding of how a concept grows from an idea, to the bench, to a clinical trial, to a marketed therapy.
CS I have recently started my PhD at Duke in Cell and Molecular Biology and am currently trying to decide on a lab and mentor for this next step.
Where will this work take the Silver lab?
DS We would like to better understand the molecular mechanisms by which Magoh dosage impairs interneuron development, including its role in splicing and translation and its direct targets, as well as discriminating why Magoh loss causes such a striking mitotic defect. It is also exciting to consider whether all EJC components act similarly in development, and how the EJC fits into a larger network as a master regulator of corticogenesis. Beyond the EJC, these studies have sparked our interest in understanding fundamental aspects of interneuron development and how genetic modifications over the course of evolution have shaped this process.
Finally, let’s move outside the lab – what do you like to do in your spare time in Durham?
JM Hiking in Eno park or exploring a new brewery.
CS Durham has a lot of great local breweries, restaurants and concerts, so I spend most of my free time exploring those with friends.
DS I spend my free time mainly with my husband, two kids and our new doggie. We enjoy the outdoors, including mountain biking in our backyard, as well as the great food and music scenes in Durham and Carrboro.


 (No Ratings Yet)
(No Ratings Yet)