A journey towards understanding the embryo – maternal vasculature interactions during implantation
Posted by Niraimathi Govindasamy, on 27 March 2022
My journey in the field of mammalian developmental biology began when I joined the lab of Dr Ivan Bedzhov at the Max Planck Institute for Molecular Biomedicine (MPI-MB) in 2016 to pursue my doctoral research. After Ivan and I discussed the many important processes that take place during the peri-implantation phase of embryonic development, it was the unaddressed mechanisms by which the embryo forms the first contacts with the mother during implantation that caught my interest the most.
The process of implantation mediates the first direct interactions between the embryo and the mother. Specialized cells of the mouse embryo, known as trophoblast giant cells (TGCs), invade deep into the uterine tissues, enabling nutrient uptake and gas exchange with the maternal environment. In turn, the uterine stroma rapidly proliferates and completely engulfs the implanting embryo. Thus, studying the process of implantation is fundamentally challenging, as the embryo is concealed by the uterine tissues and its development depends on the maternal support.
According to clinical reports, almost half of the human pregnancies fail at the time of implantation (Boomsma et al., 2009; Koot et al., 2012). However, the cellular mechanisms of implantation and the factors causing termination of pregnancy are very poorly understood. The significance of these open questions and the excitement to understand the first interactions between an embryo and the mother motivated me to work on this project.
A glimpse at the trophoblast – maternal vasculature interactions in utero
As an early miscarriage can happen because of poor trophoblast penetration and/or inadequate blood supply to the implantation site (Klauber et al., 1997; Reus et al., 2013; Torry et al., 2007), we were keen to understand whether an active crosstalk between the TGCs and the maternal blood vessels takes place during the implantation stages. We examined the spatial organization of the TGCs and determined that the invasive trophoblast is organised as strands of cells penetrating the uterine stroma. Moreover, we found that the TGCs of the implanting embryo intermingle with the surrounding blood vessels (Fig. 1). As static images were insufficient to study the dynamics of these interactions, we decided to establish a 3D biomimetic platform that resembles the biomechanical properties of the uterine stroma.
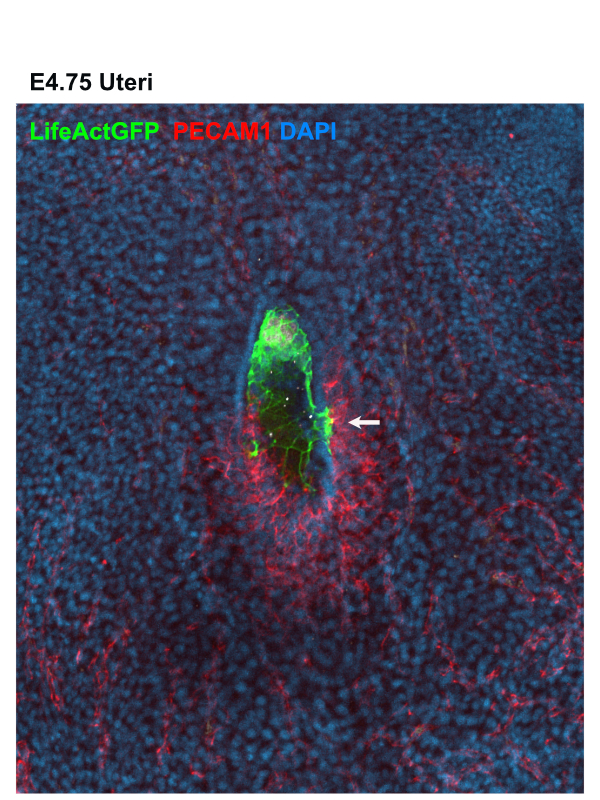
Establishment of the 3D biomimetic platform
First, we determined the stiffness of the maternal tissues using atomic force microscopy (AFM) and examined the cell adhesive properties of the endometrium. As we also had to take into account that the TGCs secrete matrix metalloproteinases (Zhu et al., 2012), we used biodegradable synthetic hydrogels as our artificial substrate. We tuned the stiffness and adhesive features of the hydrogels to mimic the biomechanical properties of the uterine stroma, (in collaboration with Dr Britta Trappmann’s and Dr Adrian Ranga’s labs). Culturing embryos in this 3D environment enabled us to directly observe ex utero implantation of the embryos for the first time, which was very exciting!
As our main focus was to understand the crosstalk between the invading TGCs and the maternal vasculature, we teamed up with Hongyan Long from Dr Britta Trappmann’s lab to incorporate the implanting embryos into a microfluidic device (Trappmann et al., 2017) which allowed us to model the interactions with the blood vessels. After several months of tireless efforts and optimization, we managed to establish the right conditions for co-culturing embryos and endothelial cells in the microfluidic chip. Using this platform, we found that the invasive trophoblast migrates towards the blood vessels to form direct cell-cell contacts, similar to the static images in vivo (Movie 1). This was truly fascinating and a very rewarding moment for us.
Uncovering the molecular basis of the trophoblast – vasculature interactions
Next, we wanted to examine the signalling crosstalk that mediates these interactions. We are grateful to Dr Hyun-Woo Jeong from Professor Dr Ralf Adams lab, who helped us with the bioinformatic analysis. We found that the TGCs gain expression of cell surface receptors, ligands and adhesion molecules, similar to the ones expressed in the nearby blood vessels. By functionally examining the main players in this process, we found that platelet-derived growth factor receptor (PDGFR) signalling promotes the establishment of direct cell-cell contacts between the TGCs and the vasculature. As PDGFR signalling has been previously shown to mediate the recruitment of pericytes to the endothelial cells (Lindahl et al., 1997), our findings suggested that the TGCs exploit this signalling pathway to locate the endothelial cells of the maternal blood vessels. Consequently, the expression of compatible cell adhesion molecules in the TGCs and the vasculature, such as VE-cadherin, enables the formation of heterologous cell-cell contacts. This discovery also has a potential clinical relevance, as it has been shown that the PDGFR inhibitor used for the treatment of chronic myeloid leukaemia increases the risk of miscarriage in experimental animals and pregnant patients (Salem et al., 2019; Ault et al., 2006; Pye et al., 2008) and the reason for this side effect was so far obscure.
Bumps along the way
Like most of the projects in academic research, the road of our journey to understand the embryo maternal interaction was not always smooth. We did face unfortunate situations, but at the same time we also got a lot of positive comments from colleagues around the world about the novelty of the project and this encouraged us that we were on the right path. Most of the methods used in this study required intense optimization. We did get stuck sometimes but we kept trying and found new ways to overcome technical limitations, which also helped me to expand my skills and critical thinking. I am really thankful to all our collaborators for their time to discuss and troubleshoot challenging experimental approaches. We learned a lot through failed experiments and eventually when we look at the new discoveries that we’ve made, I think it was all worth the effort!
When we submitted our work to two of the top journals, we experienced major delays as reviewers that accepted the invitation to review our manuscript did not respond and kept us waiting for several months. At this point we had no choice but to move to another journal, as we were running out of time and funding. This happened twice and we lost 9 months just waiting for the initial review, despite the best efforts of the editors of these journals. Having to wait so long was very stressful. Unfortunately, other colleagues have also had similar experiences, so it is up to us, the research community as well as the journals to prevent such toxic behaviour. We finally found a good home for our manuscript in Developmental Cell, where the editors are doing their best to ensure fair peer-review process and present exciting new research to the community.
In the End…
Looking back, this journey has taught me that passion, compassion, clear objectives, perseverance, and interdisciplinary collaborations are key for making new discoveries. Looking forward, I hope that one day, our findings will help scientists understand how the human embryo establishes its first direct contacts with the mother and will lead to therapeutic approaches that substantially decrease the risk of an early miscarriage.
Access the article –Niraimathi Govindasamy, Hongyan Long, Hyun-Woo Jeong, Ratish Raman, Burak Özcifci, Simone Probst, Sebastian J Arnold, Kristina Riehemann, Adrian Ranga, Ralf H Adams, Britta Trappmann, Ivan Bedzhov. – 3D biomimetic platform reveals the first interactions of the embryo and the maternal blood vessels. Dev Cell. 2021 Dec 6;56(23):3276-3287.e8.
References:
Ault, P., Kantarjian, H., O’Brien, S., Faderl, S., Beran, M., Rios, M.B., Koller, C., Giles, F., Keating, M., Talpaz, M., et al. (2006). Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol 24, 1204-1208.
Boomsma, C.M., Kavelaars, A., Eijkemans, M.J., Lentjes, E.G., Fauser, B.C., Heijnen, C.J., and Macklon, N.S. (2009). Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod 24, 1427-1435.
Klauber, N., Rohan, R.M., Flynn, E., and D’Amato, R.J. (1997). Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med 3, 443-446.
Koot, Y.E., Teklenburg, G., Salker, M.S., Brosens, J.J., and Macklon, N.S. (2012). Molecular aspects of implantation failure. Biochim Biophys Acta 1822, 1943-1950.
Lindahl, P., Johansson, B.R., Leveen, P., and Betsholtz, C. (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242-245.
Pye, S.M., Cortes, J., Ault, P., Hatfield, A., Kantarjian, H., Pilot, R., Rosti, G., and Apperley, J.F. (2008). The effects of imatinib on pregnancy outcome. Blood 111, 5505-5508.
Reus, A.D., El-Harbachi, H., Rousian, M., Willemsen, S.P., Steegers-Theunissen, R.P.M., Steegers, E.A.P., and Exalto, N. (2013). Early first-trimester trophoblast volume in pregnancies that result in live birth or miscarriage. Ultrasound Obst Gyn 42, 577-584.
Salem, W., Li, K., Krapp, C., Ingles, S.A., Bartolomei, M.S., Chung, K., Paulson, R.J., Nowak, R.A., and McGinnis, L.K. (2019). Imatinib treatments have long-term impact on placentation and embryo survival. Sci Rep 9, 2535.
Torry, D.S., Leavenworth, J., Chang, M., Maheshwari, V., Groesch, K., Ball, E.R., and Torry, R.J. (2007). Angiogenesis in implantation. J Assist Reprod Genet 24, 303-315.
Trappmann B., Baker B.M., Polacheck W.J., Choi C.K., Burdick J.A., Chen C.S. Matrix degradability controls multicellularity of 3D cell migration. Nat. Commun. 2017; 8: 371
Zhu, J.Y., Pang, Z.J., and Yu, Y.H. (2012). Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol 5, e137-143.


 (9 votes)
(9 votes)
Thank you for sharing very good post
Thank you appreciating the post!
Interesting