BSDB Gurdon Studentship Report – Anna Granés
Posted by Anna Granés, on 21 December 2022
The making of colorful neuromesodermal progenitors
During the embryonic development, while the gastrulation process is taking place, cells within embryos self-organize by creating groups and layers of cells, where each one will develop into different adult tissues. Human and mouse embryos are called triploblastic organisms as they are characterized by the development of three germ layers: ectoderm, which will form epithelial and neural tissues; mesoderm, which will differentiate into muscle, bone, circulatory system and spleen, among other tissues; and endoderm that will produce organs such as gut, lungs, and pancreas.
During gastrulation, when the germ layers are being organized, progenitor cells commit to the generation of one of these three structures and their specific cell types. For example, an ectodermal progenitor will be committed to the generation of either epithelial or neural cell types. However, some studies have described the presence of dual-fated progenitors that could produce both mesodermal and neuroectodermal lineages (Tzouanacou et al., 2009; Wymeersch et al., 2021). These cells were named neuromesodermal progenitors (NMPs) and they are identified by the co-expression of Sox2 and Brachyury (T) genes.
NMPs have risen a lot of interest due to their exceptional behavior and they are being studied with the aim to increase our knowledge about cell fate and cell differentiation, both in developmental biology and biomedical research (Binagui-Casas et al., 2021). Therefore, there has been a lot of effort on the development of in vitro models of these cells, as an easier tool to study their characteristics.
These in vitro NMP-like cells can be risen from Mouse Embryonic Stem Cells (mESC) using protocols such as Gouti et al., 2014, whose outcome has been described to be around an 80% of NMPs, within a mix of other cells in various states of differentiation. In Figure 1 we can see an example of these cultures where in vitro NMP-like cells are identified with Sox2 and T co-expression while there is also the presence of cells committed towards mesodermal lineages, identified with single T expression.
These cultures have been able to then produce both mesodermal and neural lineages at the population level, where mesodermal lineages are identified with single T expression, while neural lineages are characterized by single Sox2 expression. However, there has been a lot of controversy on whether these in vitro progenitors are also dual-fated at a single cell level as described for in vivo NMPs (Tzouanacou et al., 2009). This means that, in a group of in vitro NMP-like cells, some could be more committed to neural lineages while others to mesodermal, and it is not known yet if a single progenitor can generate both lineages.

To better characterize the potency and other characteristics of these in vitro NMP-like cells, Prof Wilson’s lab developed a new double reporter mESC line for Sox2 and T expression. In this case, mCherry protein will report Sox2 expression while GFP protein will report T expression. The knock-in experiments were performed by using gene targeting, to optimize the fidelity of expression, while leaving the endogenous gene’s expression intact. The use of this cell line allows the possibility to obtain a pure population of in vitro NMP-like cells that are double positive for Sox2 and Bra expression by using Fluorescent-activated Cell Sorting (FACS).
During my summer internship in Prof Wilson’s lab and under the supervision of Dr Anahí Binagui-Casas I have been characterizing this new cell line by describing the differentiation outcome of a pure population of in vitro NMP-like cells when cultured in different conditions and plated at different cell densities.
Firstly, in Figure 2 we can see the culture of a pure population of in vitro NMP-like cells in media supplemented with retinoic acid (RA) and smoothened agonist (SAG), as described in Gouti et al. 2014, to induce spinal cord differentiation. We can see the formation of axons and neural rosettes, and they can be identified with Sox2 expression and depleted expression of T, suggesting neural differentiation.
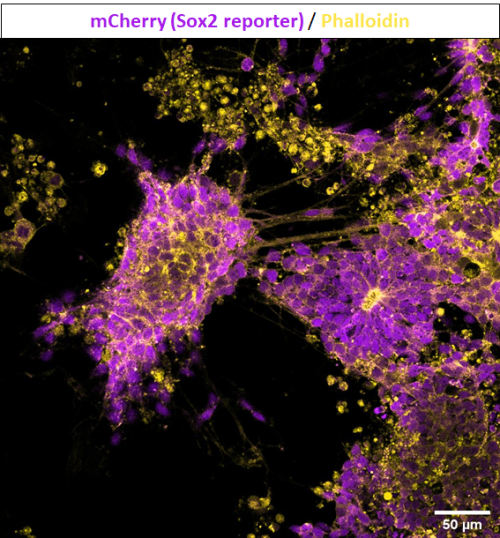
Figure 3 represents another condition where positive cells for Sox2 and Bra were cultured in media supplemented with FGF and CHIR for five days, as described in Tsakiridis & Wilson, 2015 to induce both neural and mesodermal lineages. In this case, there is the presence of cells differentiating towards neural tissues, assembled in rosette-like structures and with single expression of Sox2. Meanwhile, cells differentiating towards mesoderm are identified with single expression of T, or its gene-expression reporter GFP.
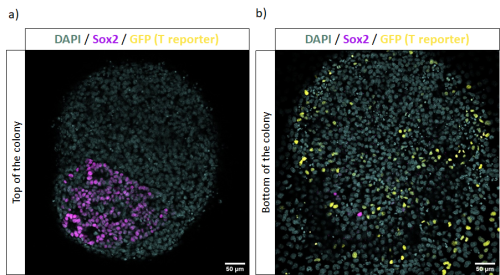
These experiments gave us insight about the differentiation outcome of this new cell line towards different lineages by testing multiple protocols. Furthermore, I was able to characterize the culture of in vitro NMP-like cells plated at different cell densities after sorting them using FACS. This knowledge will contribute to future experiments requiring the use of this double reporter mESC line as a tool to characterize in vitro NMP-like cells’ clonal fate and to understand if these cultures are a good model for in vivo NMPs.
After all this work testing multiple conditions for in vitro NMP-like cells cultures and optimizing their growth, I found out that I really enjoyed working with stem cells and differentiation protocols. Although I spent many hours in the cell culture facility losing track of time, it was very exciting to see how cells grew and changed every day. Specially, I was very excited to see how they were able to organize themselves and create amazing structures, which I then learned how to immunostain and obtain beautiful images under the confocal microscope.

To conclude, this studentship and Prof Wilson’s lab have been a great opportunity for me to learn a lot about how biomedical research is driven and to confirm that I would like to continue developing my career in developmental biology. I would like to encourage students to do these internships because it is an enjoyable way of starting to apply the knowledge acquired during your bachelor’s degree and to investigate if you would enjoy developing your career in this field.


 (No Ratings Yet)
(No Ratings Yet)