From Shifting Skies to Toxic Tides: A Tale of Flies & Flux #MetabolismMondays
Posted by Shefali Shefali, on 12 May 2025
All the world’s a metabolic dance, early career scientists are leading the way!
Emerging perspectives in metabolism

This week we will get to know insights from
Dr. Lautaro Gandara, who is a postdoctoral researcher in the Crocker lab at EMBL Heidelberg. Lautaro’s work delves into the profound interplay between metabolism, toxicology, and development. As he prepares to establish his own lab, Lautaro
is driven by a deep curiosity about how life adapts to environmental challenges, and how metabolic shifts shape the very essence of biological resilience. From his studies of Drosophila melanogaster to the impact of environmental stressors on insect populations, his research questions the fundamental nature of life’s response to stress and transformation. For Lautaro, science is not just a pursuit of answers but a journey of discovery, where each question unfolds new dimensions of understanding. He believes that the study of metabolism and development is not merely academic—it’s a window into the intricate ways life connects, adapts, and evolves. Follow his journey as he continues to explore these deep questions and check out his work here.
Could you share your journey into studying metabolism and what inspired you to specialize in metabolic studies using Drosophila melanogaster.
When I took an introductory course in chemical biology as an undergrad, I remember being less than enthusiastic about the field. Metabolism was presented as a completed research program—a field in which human metabolic maps had already been established, flux control theory had provided all the relevant dynamical information, and the only open questions were clinical ones. It was only after I started my PhD work in Pablo Wappner’s lab that I got access to the then-new research showing how metabolism, far from being a housekeeping process, can actually transmit information by regulating gene expression, signaling pathways, and so on. At the time, I was studying the response to oxygen deprivation (hypoxia) in flies, and we soon realized that many metabolic facets of this process remained unexplored. Drosophila larvae can perform lactic acid fermentation in hypoxic environments, but at that time there was little information on the spatial and temporal properties of this metabolic switch. These questions became the focus of my work as a grad student.
How did you get interested in the field of toxicology and impact of chemicals on insect health and metabolism? How do you think toxicology and metabolism fields overlap and how they regulate each other or how they are connected?
I have always been fascinated by the way in which life actively responds to environmental change—its intrinsic ability to preserve itself by regulating its own activities and structures. When you look at these processes more closely, all the reductionist metaphors of “life as mere machines” start to crumble, right? This is where my interest in the hypoxia response originally came from. So as a postdoc in Justin Crocker’s lab at EMBL, I wanted to expand on the approaches I had used as an undergraduate to further explore these phenomena. Instead of focusing on just one environmental perturbation (hypoxia) and one phenotype (metabolism), we decided to test more than 1000 different chemical stressors and measure how the effects induced by these molecules propagate across the different scales of biological organization.
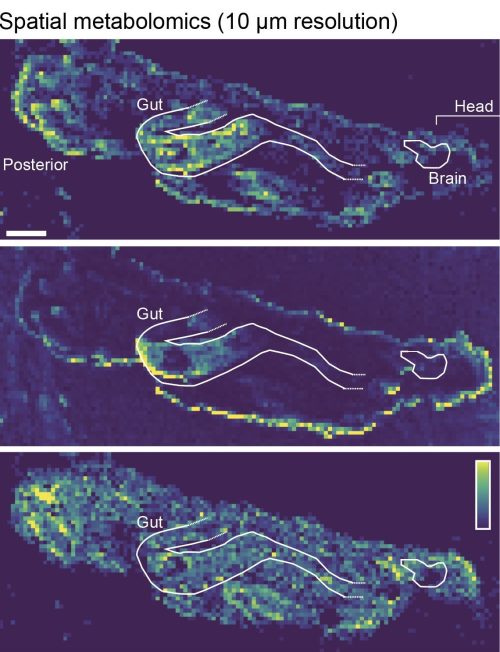
177.0158 (C7H6O4); lower panel =744.5537 (C41H78NO8P). Image source: LG.
Tell us about how you see the future of metabolism evolve with the new upcoming tools like the FRET biosensors you worked on. What changes have you seen in the community regarding exploring metabolic aspects of development ?
I consider the development of tools to be an
essential driver of science, and I think that the interplay between development and metabolism is a paradigmatic case of this phenomenon. In the first half of the 20th century, embryologists such as Joseph Needham studied the metabolic facets of development using the technology available at the time (calorimetry, respirometry, etc.). However, as embryology was transformed into developmental biology, and the focus shifted from organismal-level processes to gene- and cell-level phenomena, this set of tools proved ill-suited for exploring metabolism at this scale. As a result, questions about the role of metabolism during development were put on hold. Recently, the combination of metabolic FRET sensors, spatial omics techniques (especially spatial metabolomics), and flux analysis by isotope tracing has reignited interest in these old questions and revitalized the field. Ongoing research by many different groups working on different model systems around the world is providing priceless information about the precise role of metabolism in the development of multicellular organisms.
One of your reviews is titled “Metabo-Devo: A metabolic perspective of development”. Could you elaborate on the key findings and their implications for the field? How do you integrate different disciplines – metabolism, development, and evolution in your research, and what unique insights have emerged from this approach?
In that review article, we proposed a conceptual framework to discuss how metabolism interacts with developmental processes. We classified these interactions as either 1) bioenergetic functions, 2) regulation of gene expression through changes in the epigenome, or 3) signaling functions.
Bioenergetic processes are those that provide energy or building blocks to developing tissues. Many cell populations that proliferate at high rates acquire a particular metabolic state, called aerobic glycolysis, that allows them to synthesize macromolecules at the right pace. This metabolic switch was reported ~100 years ago in the context of cancer biology (i.e. the Warburg effect), but it is now becoming clear that aerobic glycolysis is also required for cell proliferation in developing organisms. Ongoing research efforts aim to elucidate the developmental processes during which this metabolic transition occurs, and how it is regulated.
In addition to this bioenergetic role, specific metabolic pathways can directly regulate gene expression. Certain metabolites have been shown to act in developmental contexts as rate-limiting substrates for histone and DNA modification. And this metabolic control of gene expression has been shown to play an essential role in key developmental processes, such as the zygotic genome activation. Similarly, many metabolites are directly involved in the post-translational modification of signaling-related proteins, while several metabolic enzymes have been reported to act as multifunctional “moonlighting” proteins that can perform alternative functions not necessarily related to metabolism. Thus, metabolites and metabolic enzymes have the potential to modulate signaling pathways that are essential for development.
Although we think that the classification described above provides a useful conceptual framework for designing and discussing experiments, dissecting the actual role of metabolism in specific processes remains challenging, especially because the same metabolites and enzymes may simultaneously play bioenergetic and signaling roles that affect the same developmental phenomenon. In any case, the emerging view is that metabolism and development are deeply intertwined processes that cannot be disentangled. Thus, this observation highlights the need for a discipline or research area—developmental metabolism or “metabo-devo”—that directly addresses these issues.
You are currently studying the impact of toxic substances from genotypic to phenotypic perspectives and your work suggests the use of agrochemicals is the root cause of insect decline. Can you briefly discuss this work ?
We decided to focus on agrochemicals (i.e. insecticides, fungicides, plant growth regulators, etc.). Increased use of pesticides has been proposed as a potential cause of the widespread declines in insect populations, but studies investigating the effects of these molecules on insects are often limited to a few chemicals and a single insect species. We started with a screen that tested the effects of 1024 agrochemicals on the behavior of Drosophila larvae. Behavioral changes often have a mechanistic basis at simpler phenotypic levels, and thus monitoring behavior can provide important information about the state of the biological system as a whole. Surprisingly, we found that ~60% of the molecules in our library significantly alter larval behavior! And these effects are not limited to flies—we also detected similar sublethal behavioral changes in mosquitoes and butterflies, suggesting a generalizable effect on insect populations.
By exploring some of the screen hits further, we found that the effects go well beyond behavior. Exposure to sublethal doses triggered widespread phosphoproteomic changes, revealing how these chemicals affect many different physiological processes. And chronic exposure led to delayed development and reduced reproductive output, potentially contributing to the decline in insect populations. Thus, the study showed that even non-insecticidal pesticides at field-realistic sublethal concentrations can have profound ecological consequences, highlighting the need for better safety assessments that take sublethal effects into account.
How difficult some of those experiments work – did you have to deal with midnight timepoints or require an army of undergrads/ long hours ?
The behavioral screen was very time consuming. We ended up testing 3072 different conditions (different molecules and concentrations), so including replicates and controls, we ran more than 10000 individual assays… It was a lot of work, but I got a lot of help from everyone in the lab, and fortunately we managed to get it done in just a few months. It was truly a collaborative effort!
Can you shed light on the big picture of the field, what are you most excited about and how does it all connect to impacting insect/human health.
Understanding how animal systems respond to stress is becoming increasingly important in the current context of human-induced global environmental change. Going back to my fascination with the resilience of biological systems, I think our previous work has opened up an exciting opportunity to test some of the open questions in the field of stress response. There is this idea that multicellular organisms need to activate a system-level stress response to restore homeostasis. This process would be based on the well-known “integrated stress response” pathway—a process that occurs at the cellular level—but would also involve organismal level stress defense systems involving cell differentiation processes, metabolic switches, physiological changes, and even behavioral effects. Testing this hypothesis, however, is not straightforward because it would require measuring the degree of interconnectedness among all these different processes across a wide range of environmental perturbations. By performing the behavioral screen I mentioned earlier, we have defined a panel of chemicals that induce widespread systemic changes in fly larvae, but at sublethal concentrations, meaning that these animals can orchestrate a successful response to these stressors and recover from these injuries. Thus, this panel of chemicals can be used next to explore the level of integration between the different stress defense systems operating at the organismal level. I hope to start my own group soon, and this is one of the first problems I’d like to tackle.
What advice would you offer to students and early-career scientists interested in exploring the intersections of metabolism, development, and evolution?
I’d tell them to have fun! These are indeed exciting times to be doing metabolic research in developmental systems. New technologies are allowing us to explore the various ways in which metabolism transfers information not only across space (inter-organ communication, metabolic coupling between cell types, etc.), but also across time (developmental and cell differentiation processes). I think this is the time to be bold and creative in finding ways to make the most of this technological advantage.
What role does curiosity play in your life, both within and outside of science? How important is it for you to answer basic science questions about behavioral and metabolic aspects of toxicology and how are you planning to use insect models to bridge basic science and applied research ?
Curiosity does play an essential role in my life and in my research. The project I mentioned earlier, in which we studied the sublethal effects of agrochemicals on insects, has some facets that are obviously relevant to the community as a whole. But I strongly disagree with the idea that it’s only worth studying certain natural phenomena if they affect us directly, or if we can use them for our direct benefit in the short term. Basic and applied research aren’t in opposition to each other—on the contrary, they are involved in a dialectical feedback in which the former feeds the latter with information about how fundamental processes work, while the latter not only highlights which questions are most pressing, but also drives the development of new tools and methods that can then be applied to basic research. Hypoxia research is a good example. The oxygen sensor—the molecule that allows cells to determine oxygen availability and trigger an appropriate response when oxygen levels become too low—was identified more than 20 years ago in fundamental work on C. elegans. Years later, this molecular machinery was found to have enormous clinical relevance, as it could be manipulated to induce angiogenesis and treat the symptoms of cardiovascular disease or prevent it and limit tumor growth. But this useful knowledge first came from curiosity-driven research on basic genetics.
Were there any pivotal moments that shaped your career path? What’s an unexpected place you’ve found inspiration for your work?
It may sound simple, but for me the most important source of scientific inspiration is talking to other people. I can think about some problems for hours, but in my case, ideas really take shape when I express them, either by writing them down or by discussing them with someone else. It’s as if, by trying to communicate my thoughts, I organize them into a coherent narrative—a logical structure—from which new ideas can occasionally emerge. And then different people, with different backgrounds and different opinions, will steer your train of thought in completely different directions, certainly leading to unexpected places… But I’m not talking about big meetings full of people here—it’s the one-on-one discussions that force you to interact longer with a single concept and exhaust all its multiple possibilities that are often more productive for me.
If you hadn’t embarked on a career in biological research, what other profession might you have pursued, and why?
I don’t really see myself doing anything other than biological research. But if it weren’t for biology, I think I would have pursued a career in the social sciences. Human societies are incredibly complex entities, but understanding how they work is not just a matter of academic curiosity. Especially now, looking at the current times, explaining how society, economics, or history actually works has become a pressing issue. I think that on an individual level—and as citizens —we can’t afford to ignore these problems any longer.
Anything you’d want to highlight for the future.
I’m currently looking for a place to set up my own lab. Aside from the global uncertainty we discussed earlier, I think these are exciting times to start a group. New technologies are making old questions experimentally tractable, new species are being proposed all the time as novel model systems, and AI promises to change the way we approach data analysis. There’s no doubt that the way we do science is going to change dramatically in the coming years, and that’s an idea I find particularly appealing.
Last week we learnt about how males and females are metabolically differently rewired – from the perspective of lipid storage and utilization – The Fat of the Matter (Lianna Wat)
Check out the article All the world’s a metabolic dance, and how early career scientists are leading the way !!


 (5 votes)
(5 votes)