Hybrid brains and the search for what makes us human
Posted by Caleb Gordon, on 28 May 2025
It lives. It lives! What lives, you may ask? Well, somewhere in a lab at Yale University, one young scientist has stuck human brain cells and chimp brain cells together to make a chunk of hybrid brain. A few weeks ago, I met with her to ask more about this research. She admits that it all sounds like “mad science,” but this mad scientist might be taking a big step forward on our path to find out what really makes us human.
Chimpanzees are our closest relatives, and closer than most of us would probably like to think about. We share some 98.8% of our DNA with chimps.1 This means only about 1.2% of our DNA accounts for the uncanny power of our species to build cities, write symphonies, split atoms, and do all the other things we alone do so well. We know that much of this uncanny power resides in our brain, which is massive compared to a chimp’s brain,2 and has a much larger wrinkled region at its front3 that does most of our complex “higher” thinking. This wrinkled region at the front of our brains takes almost twice as long to finish developing in us as it does in chimps,4 and scientists have long thought that its slow development in humans helps to explain our subtle and adaptable “higher” thinking abilities.4 What we do not know is how that meager 1.2% of our DNA goes about making our wrinkly-fronted brains develop so slowly.
For the last two years, a young scientist named Reem Abu-Shamma has been trying to change that. Since graduating summa cum laude from UCLA, Reem has made a career of mutating genes, creating artificial 3D clusters of human intestinal cells (delightfully called “organoids”), and using computer programs to study vast amounts of DNA. These endeavors might sound eerily sci-fi, but have in fact taught us a lot about public health and disease. Her work mutating genes in parasites could shed light, down the line, on how we treat some particularly nasty strands of malaria,5 and her work with human intestinal organoids promises to tell us more about the cellular basis for inflammatory bowel disease. Now, as a PhD student at Yale University, Reem has set her sights on what makes our brains human.
” Slower development means more time to make a big brain…So where in the genetic code is it telling our brains to develop slower? “
To investigate what makes our brains unique, Reem has created something like a hybrid “half-human, half-chimp brain.” This phrase baffled me as much as it’s probably baffling you, so I sat down with Reem to ask her more about what inspired this research. And, as I listened to Reem’s enthusiastic, down-to-Earth explanation for her project, it began to seem less like mad science and more like vital research. “Large brains allowed us to dominate the world for better or for worse,” Reem explains. She wants to find “the underlying code in our cells that has allowed us to do that.” In searching for this code, Reem has focused on the speed at which that wrinkly portion at the front of our brains develops. “Slower development means more time to make a big brain…and we know that the human brain takes a really long time to develop.” This slow pace of human brain development manifests at the cellular level6—individual human brain cells take years to branch out and mature, whereas those of chimps develop much faster. Reem’s research question is simple, then: “Where in the genetic code is it telling our brain cells to develop slower?”
To answer this question, Reem recently joined the Noonan Lab at Yale University, which has a long history of using the best-available gene-editing technology to study human brains. One particular focus of the Noonan Lab has been to find particular bits of DNA that distinguish humans from chimps and other animals. What exactly are these bits of human specific DNA? Well, as Reem explains, “they’re parts of the genome that are not genes,” but “dials” for genes, which make various brain-building genes more or less active as the brain develops.7 These bits of DNA are part of that 1.2% of our genetic code separating us from chimpanzees, and could tell us a lot about how that huge wrinkly portion at the front of our brains develops so slowly, gets so big and complex,5 and makes us so clever. Each one of these bits, once found, “gives us a hint that maybe this part of the genome helped us evolve big brains.” However, that hint alone doesn’t prove the bit’s role in making our brains bigger or tell us how it did. In order to actually verify what these human bits of DNA do, scientists have to mutate them, and see how those mutations affect the development and interaction of different human and chimp brain cells over time. Obviously, no one at any credible research institution wants to mess with the brain of an actual living human—institutional and federal guidelines fortunately forbid that kind of work. But scientists dowant to understand what these human-specific bits of DNA are doing. So how do you mutate realistic human brains without using actual real human brains?
Well, remember those “organoids” I mentioned before? Reem uses those. And they’re a lot less scary than they sound. “We’re not using real animals, or growing real brains” Reem assures me with a laugh. Instead, she’s using what amounts to just a few cells: To create brain “organoids”—again, 3D clusters of living brain cells—she uses cells that other labs have collected from human or chimp skin. These labs treated those skin cells with various molecules to “reprogram” them into stem cells, which can turn into almost any other kind of cell if given the right molecular cues. “In our case,” Reem explains, “we make them turn into neurons.”8 Reem uses this approach to create human and chimp brain cells, and then grows each reprogrammed brain cell into a different 3D cell cluster or “organoid.” The result in each case is a separate ball of brain cells9 for each species that develops much like they would in a real brain.
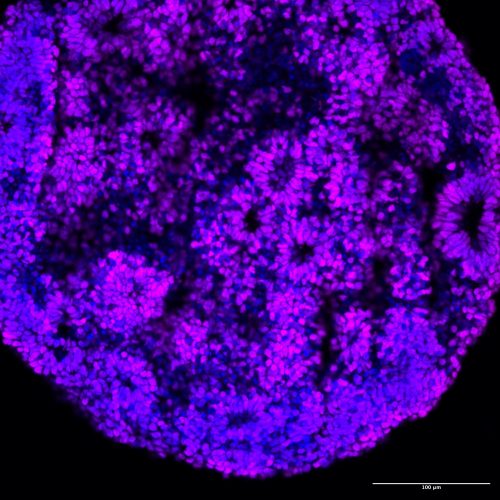
A human brain organoid, 30 days old, made by combining two different human cell lines. Cells are labeled with two overlain molecular markers——a blue one marking all cell nuclei and a violet one that marks “forebrain cortical neuron progenitors” (the kind of cells that end up forming the wrinkly front of our brains). The cells in this organoid have spontaneously arranged themselves into “rosettes”, much like brain cells do in an embryonic brain. Image courtesy of Reem Abu-Shamma.
With each brain organoid, Reem plans to test what our human-specific bits of DNA are doing to make our brains grow slower and larger. She will do this by tweaking or changing10 various human-specific bits of DNA to make them act more like the corresponding regions of chimp DNA, and vice versa. Then she’ll see how these modifications affect the activity levels of various brain cell genes and the “speed” at which those brain cells ultimately develop. “By ‘speed’, we don’t mean absolute time; rather, we have the technology to look at a single cell and figure out how mature it is based on the molecules we observe in it,” Reem clarifies. Then, for each bit of human-specific DNA, she’ll see whether the humanized chimp cells appear to develop more slowly, while the “chimpanized” human cells develop more quickly. Then we would know that this specific bit of our 1.2% unique genetic code is partly responsible for making our brains so weirdly human.
Reem finds the sheer size of this mystery fascinating. “The genome is a really big place,” she explains. “It’s so vast and we don’t know what most of it does. It kind of feels like detective work, because you’re trying to see where in this really big space it’s telling us to be human.” By tweaking little bits of human and chimp DNA so they behave more like their counterparts—a sort of genetic Freaky Friday—Reem can do just that, finding which bits of human-specific DNA tell our brain cells to grow in a human way. This in itself is the stuff of science fiction. However, Reem and her PhD advisor, Dr. James Noonan, are taking this approach one step further.
They aren’t just growing human brain-cell colonies and chimp brain-cell colonies. They’re mixing them together, to make something like a miniature hybrid brain. Despite their different origins, these cells branch out and interconnect much like the cells in our own brains, possibly creating a cellular communication network unseen in nature. “Why would you make a half-human, half-chimp brain?” Reem jokes that her mother and even her colleagues have often asked her this question. But Dr. Noonan initially suggested this approach, and Reem has pursued it, because we can learn a lot from it.
” It kind of feels like detective work, because you’re trying to see where in this really big space it’s telling us to be human. “
Brain cells don’t usually grow on their own. They grow in response to cues from neighboring cells, and these hybrid brains can show us the extent to which human brain cell development is genetically encoded. How much of how our brain cells behave is written in their DNA, and how much is determined by interaction with their cellular neighbors? Specifically, Reem is curious whether the sum of brain cell interactions, and the presence of similar brain cells from other species, together affect how fast that wrinkly portion at the front of the brain develops. Previous studies have found that these external cues (the “cellular environment”) don’t matter much for the development speed of human brain cells.11,12 However, few if any studies have used hybrid chimp-human brain organoids to study that big wrinkly-fronted part of the human brain. By creating hybrid chimp-human organoids with this specific type of brain cell, Reem will finally test whether environmental cues help it grow slower in humans. Reem gives me an example to help me wrap my head around this. So, suppose you “take a human cell and transplant it into a chimp brain organoid,” Reem explains. And then suppose you collect molecular data from human brain cells inside a purely human organoid and then do the same to human brain cells inside a human-chimp hybrid organoid. “If they’re exactly the same, then the environment the cell is in isn’t as important!”
Making and mutating hybrid brains is intense work. To do it right, Reem has to set up hundreds of different brain organoids, each in its own plastic well, and from a variety of different human and chimp donors. She goes into the lab every day. “I check on my cells immediately…first thing. I make sure they’re still alive.” She recounts instances where some of her organoids became cancerous, and others spontaneously collapsed and started dying—both unplanned events that threatened to skew her work and required hours of manual labor to remedy. “So many things can go wrong…it’s a lot of manual labor to make sure they’re alive and happy.” On a daily or weekly basis, she has to feed her many hundreds of brain organoids, and look at each one under a powerful microscope to make sure nothing has gone horribly wrong. She has to modify them all at just the right time, in just the right way. And then, when all of that is done, within the next few months, she’ll have to extract specific molecules from these organoids and analyze the resulting vast amounts of data to see how her mutations changed the approximate speed of brain cell development in her human brain, chimp brain, and hybrid brain organoids.
Reem is eager to find “what inferences we can make about the speed of development using these models”—at what rate the brain cells are likely growing, dividing, branching out, and developing their various special functions. With this approach, Reem wants to pinpoint some of the intrinsic genetic factors responsible for speeding up and slowing down the “molecular rate” of human brain cell development.
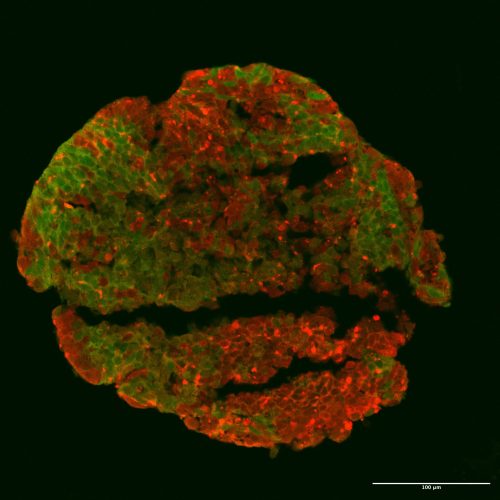
A hybrid human-chimp brain organoid from Reem’s first experiment on this project. Molecular markers tag chimp brain cells red and human ones green. This hybrid chunk of brain is 17 days old. Image courtesy of Reem Abu-Shamma.
When I asked Reem about the benefits of this research, her answer surprised me. Of course, her work does have implications for treating and understanding psychiatric or developmental conditions—autism spectrum disorder, schizophrenia, and other cognitive differences that often relate to brain development. That was the answer I expected. But Reem went on to highlight something else. “This is a very exploratory study,” she explained. “It’s hypothesis-generating,” and “in the history of science, doing fundamental research can sometimes lead you down unexpected paths, just because you’re exploring your curiosity.” This “fundamental research” is done not for its direct societal benefits, but to better understand ourselves and our world, and often has unexpected humanitarian value. For example, Reem points out, CRISPR was discovered by fundamental research projects on a few seemingly random repetitive patterns in microbe DNA. And yet, CRISPR now forms the most promising avenue for therapeutic gene editing and has a variety of other applications for human health and disease worldwide.13,14
Reem’s work on hybrid brains is fundamental research in the same way. Yes, it has biomedical implications. But its potential value is so much broader. It can shed light on the parts of our genetic code that separate us from chimps and other animals. As Dr. Noonan told her when he first suggested making human-chimp hybrids, “no one’s done it before,” and we can hardly begin to predict what it might tell us about what makes us human.
——————————————————————————————————————————————————————————————————————————————
Caleb Gordon is a Postdoctoral Associate at Yale University, where he studies the evolution of reptiles during the time of the dinosaurs. Check out his website to follow his research and popular science writing.
Note from the author: This piece was written as part of a workshop series taught by Carl Zimmer, and organized by Yale’s Graduate Writing Lab, on science reporting intended for a general audience. This workshop challenged us to write a popular science article without any scientific jargon. However, for any scientists missing this jargon, I’ve included more scientific terminology in the References Cited below. This article benefited greatly from feedback by Lauren Gonzalez and Joseph Lee at the Graduate Writing Lab.
——————————————————————————————————————————————————————————————————————————————
References Cited
- [1] The figure of 98.8% is given in Smith, Shelley L. “Connecting with Our Ancestors: Human Evolution Museum Experiences.” Interdisciplinary Evolution Research 9, 1–528. https://doi.org/10.1007/978-3-031-69429-5. It is also often shown online: American Museum of Natural History, Hall of Human Origins. “DNA: Comparing Humans and Chimps.” Accessed online April 25, 2025. https://www.amnh.org/exhibitions/permanent/human-origins/understanding-our-past/dna-comparing-humans-and-chimps.
- [2] For more information about the evolution of human brain size, you can check out this research paper: Smaers, J. B., R. S. Rothman, D. R. Hudson, A. M. Balanoff, B. Beatty, D. K. N. Dechmann, D. De Vries, et al. “The Evolution of Mammalian Brain Size.” Science Advances 7, no. 18 (April 30, 2021): eabe2101. https://doi.org/10.1126/sciadv.abe2101.
- [3] This wrinkled region at the front of the brain is called the “prefrontal cortex.” For more information about this remarkable brain region and its implications for our higher executive functioning abilities, you can check out this research paper: Preuss, Todd M., and Steven P. Wise. “Evolution of Prefrontal Cortex.” Neuropsychopharmacology 47, no. 1 (January 2022): 3–19. https://doi.org/10.1038/s41386-021-01076-5.
- [4] Additional information on the prefrontal cortex is provided in this research paper from the same journal: Kolk, Sharon M., and Pasko Rakic. “Development of Prefrontal Cortex.” Neuropsychopharmacology 47, no. 1 (January 2022): 41–57. https://doi.org/10.1038/s41386-021-01137-9.
- [5] The following research paper summarizes the results from Reem’s collaborative gene-editing work with malarial disease vectors: Subudhi, Amit Kumar, Anne-Lise Bienvenu, Guillaume Bonnot, Reem Abu-Shamma, Faryal Khamis, Hussain Ali Abdulhussain Al Lawati, Stephane Picot, Eskild Petersen, and Arnab Pain. “The First Case of Artemisinin Treatment Failure of Plasmodium Falciparum Imported to Oman from Tanzania.” Journal of Travel Medicine 30, no. 3 (May 18, 2023): taac092. https://doi.org/10.1093/jtm/taac092.
- [6] The human brain matures more slowly in part because individual human brain cells take longer to develop. For a great review highlighting the uniquely protracted nature of human brain cell development, check out this recent paper: Lindhout, Feline W., Fenna M. Krienen, Katherine S. Pollard, and Madeline A. Lancaster. “A molecular and cellular perspective on human brain evolution and tempo.” Nature 630 (19 June 2024): 596–608. https://doi.org/10.1038/s41586-024-07521-x.
- [7] For more information on what these human-specific bits of DNA are and what they do, check out this recent paper from the Noonan Lab: Pal, Atreyo, Mark A. Noble, Matheo Morales, Richik Pal, Marybeth Baumgartner, Je Won Yang, Kristina M. Yim, Severin Uebbing, and James P. Noonan. “Resolving the Three-Dimensional Interactome of Human Accelerated Regions during Human and Chimpanzee Neurodevelopment.” Cell 188, no. 6 (March 2025): 1504-1523.e27. https://doi.org/10.1016/j.cell.2025.01.007.
- [8] For additional information about how these scientists create brain cells from stem cells, check out the following paper: Mariani, Jessica, Maria Vittoria Simonini, Dean Palejev, Livia Tomasini, Gianfilippo Coppola, Anna M. Szekely, Tamas L. Horvath, and Flora M. Vaccarino. “Modeling Human Cortical Development in Vitro Using Induced Pluripotent Stem Cells.” Proceedings of the National Academy of Sciences 109, no. 31 (July 31, 2012): 12770–75. https://doi.org/10.1073/pnas.1202944109.
- [9] These colonies of brain cells are called “cortical organoids.” For more information about these remarkable 3D brain cultures, check out the following paper: Pollen, Alex A., Aparna Bhaduri, Madeline G. Andrews, Tomasz J. Nowakowski, Olivia S. Meyerson, Mohammed A. Mostajo-Radji, Elizabeth Di Lullo, et al. “Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution.” Cell 176, no. 4 (February 2019): 743-756.e17. https://doi.org/10.1016/j.cell.2019.01.017.
- [10] Reem mutates brain cell colonies using “arrayed CRISPR screens,” which are described in more detail in the following research paper: Bock, Christoph, Paul Datlinger, Florence Chardon, Matthew A. Coelho, Matthew B. Dong, Keith A. Lawson, Tian Lu, et al. “High-Content CRISPR Screening.” Nature Reviews Methods Primers 2, no. 1 (February 10, 2022): 8. https://doi.org/10.1038/s43586-021-00093-4.
- [11] This research paper studied pure human and pure chimp brain cell organoids: Otani, Tomoki, Maria C. Marchetto, Fred H. Gage, Benjamin D. Simons, and Frederick J. Livesey. “2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size.” Cell Stem Cell 18 (April 7, 2016): 467–480. http://dx.doi.org/10.1016/j.stem.2016.03.003.
- [12] This research paper took human brain cells from that wrinkly region at the front of the brain and transplanted them into a live mouse brain: Linaro, Daniele, Ben Vermaercke, Ryohei Iwata, Arjun Ramaswamy, Baptise Libé-Philippot, Leila Boubakar, Brittany A. Davis, Keimpe Wierda, Kristofer Davie, Suresh Poovathingal, Pier-Andrée Penttila, Angéline Bilheu, Lore De Bruyne, David Gall, Karl-Klaus Conzelmann, and Vincent Bonin. Neuron 104 (December 4, 2019): 972–986. https://doi.org/10.1016/j.neuron.2019.10.002.
- [13] Doudna, Jennifer A., and Emmanuelle Charpentier. “The New Frontier of Genome Engineering with CRISPR-Cas9.” Science 346, no. 6213 (November 28, 2014): 1258096. https://doi.org/10.1126/science.1258096.
- [14] Barrangou, Rodolphe, and Jennifer A Doudna. “Applications of CRISPR Technologies in Research and Beyond.” Nature Biotechnology 34, no. 9 (September 2016): 933–941. https://doi.org/10.1038/nbt.3659.


 (5 votes)
(5 votes)
Very interesting!
I have a few questions:
1) in a human chimp hybrid organoid, is there a critical number of human or chimp cells at which the hybrid functions as human, or as chimp?
2) are the cells in the organoid uniformly differentiated, or are they at various stages of differentiation?
Thank you! And good questions! I’ve just passed them along to Reem, who will answer them :)
Hi Smrithi,
1) Interesting question, which I don’t think I will fully answer in my project! We would need to define what functioning as human vs. chimp means. At the organoid level as a whole, this will need to include some functional assays (e.g. electrophysiology, calcium imaging). My readout and resolution will be on the single-cell level. So would be interesting to see shifts in expression profiles when seeding different proportions of human vs. chimp cells to make the organoids.
2) Cells in an organoid are at various stages of development, as is in a developing fetal brain!
Thank you very much for your response, Reem!