The people behind the papers: Dae Seok Eom & David Parichy
Posted by the Node Interviews, on 7 April 2017
Macrophages are usually associated with immunity, but have increasingly appreciated functions in development and homeostasis. This week we meet the authors of a recent Science paper that identified a role for macrophages in zebrafish stripe patterning, revealing a remarkable ‘relay’ mechanism whereby macrophages help one type of cell signal to another via cytoplasmic extensions. Postdoc Dae Seok Eom and his supervisor David Parichy, recently appointed Pratt-Ivy Foundation Distinguished Professor of Morphogenesis at the University of Virginia, told us more.

David, can you give us your scientific biography and the questions your lab is interested in?
DP I started out in ecology and evolution as an undergrad at Reed College, studying maternal effects on tadpole growth and survivorship for four years with Bob Kaplan. With that experience I applied to E&E Ph.D. programs and ended up accepting an offer from Population Biology at UC Davis, with Brad Shaffer, a systematist and organismal biologist studying biogeography of salamander populations. But between when I applied and when I got there, I became more and more interested in developmental mechanisms and how they evolve. So I was really lucky that Brad has diverse interests and that Carol Erickson—a developmental biologist working on neural crest—was willing to serve as a co-advisor. For my dissertation I focused on the cellular bases for salamander pigment pattern development and evolution. Those are great animals, but the molecular biology and genetics were difficult at the time. So I switched to zebrafish and its relatives for my postdoc with Steve Johnson at Wash U Medical School in St. Louis. In Steve’s lab, we identified some of the mechanisms underlying the development of stripes and other patterns, and I carried this program into my independent career.
My lab has broad interests but an organizing theme has always been to understand the genes and cell behaviors underlying adult phenotypes, and how changes in developmental genetic mechanisms contribute to variation within and among species. We continue to work on pigment patterns, but we also want to know what regulates the stem cells that give rise to adult pigment cells and how “local” cellular mechanisms intersect with “global” endocrine control during development, homeostasis and regeneration. But our interests are wide-ranging so we’ve also worked on topics including skeletal development, zebrafish natural history, and the behavioural significance and cognitive processing of pigmentation. Right now we are doing a lot of work to understand scale morphogenesis and patterning in fish, and we’ve even started working on salamanders again, both pigment and regeneration.
And Dae Seok, how did you come to join David’s lab?
DSE Actually, when I was at PhD training at the University of Texas at Austin, Dave was a faculty of my department, and I was interested in his work, but by then he was then planning to move to the University of Washington. While I was finishing my PhD training, my wife started her PhD at UW, so I had to find a postdoc position in Seattle. I realised Dave was there, and it was obviously a great second chance to work with him – and he accepted.
Aside from aesthetics, what makes zebrafish stripe development an attractive developmental model?
DP When I was a grad student, I wanted to find a system that could be studied from many different angles—molecular through organismal. And this was why I ended up focusing on pigmentation. For anyone with broad interests it’s just a natural: there’s a deep literature on pigment cells and pattern going back to the turn of the last century, the cells are visible even in the living organism as the phenotype is developing, the patterns themselves often have profound ecological significance, and there’s tremendous diversity of pattern across even closely related species. Of course there are also a variety of pigmentary disorders, including melanoma, and pigment cells develop and regenerate from stem cells—so there is obvious biomedical utility in studying pigment cells, especially with a system like zebrafish in which the genetic and cellular mechanisms are so accessible.
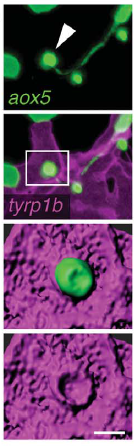
A macrophage drags an airineme to a melanocyte. Eom & Parichy, 2017.
Can you give us the key results of the paper in a paragraph?
DP We showed previously that consolidation of melanophores into adult stripes depends on interactions between these cells and xanthoblasts, the precursors of yellow xanthophores. During a specific stage of stripe development, xanthoblasts that happen to be present in future stripe regions extend very long, thin and meandering filopodia-like processes with membraneous vesicles at their tips. We called these projections “airinemes” after Iris—messenger of the gods—and Sir George Airy, who described limits on optical resolution, because the projections are such a pain to visualize. We saw airinemes “dock” with melanophores, and we found that blocking airineme extension prevented melanophores from consolidating properly into stripes, at least in part because of a defect in Delta-Notch signalling.
“Weird biology”
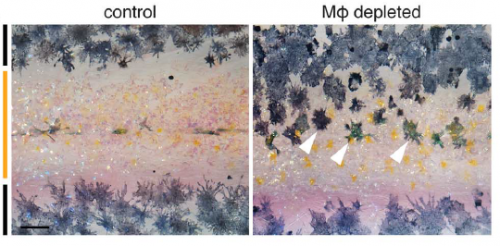
In Dae Seok’s new paper, we show that airineme extension and vesicle delivery depend on macrophages that are cruising around the local tissue environment. The macrophages recognize surface blebs on xanthoblasts and try to engulf them but continue to wander, pulling a membrane filament from the xanthoblast as they go. Eventually they wander across a melanophore and the vesicle and filament are deposited on the melanophore surface. If we get rid of macrophages, we prevent airineme extension and melanophores remain dispersed. Weird biology.
What convinced you to deplete macrophages in the first place? It might not seem like the most obvious place to look for patterning regulators…
DSE The idea initially came from the question of what would happen to unbound airineme vesicles. When xanthoblasts don’t meet the target melanophores, airineme vesicles detach from the airineme filaments and wander away. We knew the vesicles carry DeltaC and possibly other signalling molecules. Thus, these unbound vesicles should be somehow eliminated. The question then was what cell types can do that? One of the answers was the macrophage.
DP Plus we could constantly see macrophages wandering around in Dae Seok’s movies. How could we not try getting rid of them?
Is there anything in the macrophage-depleted fish to suggest that other patterning or morphogenetic events may be affected, or is this just tissue specific?
DP We’re only starting to investigate roles for macrophages as well as airinemes in other tissues. Because macrophages wander all over, you could imagine a whole variety of possibilities for diffusion-like dissemination—or regulated attenuation—of signals in other contexts. The more people look, the more interesting things macrophages seem to do.
Can you hazard a guess as to whether you think the macrophages actively select signalling targets or just wander around randomly?
DSE We have no idea at this time but my guess is macrophages constantly probe the environment, and signals from the airineme vesicles instruct macrophages to detect the targets and drop the vesicles while they are randomly wandering around.
DP Yes, because Dae Seok showed that targeting is specific to a subset of melanophores, there has to be some sort of airineme vesicle–melanophore recognition system. It will be interesting to see whether this is somehow instructive to the macrophage itself or whether the macrophage is simply trying to eat the vesicle and can’t manage to do so, and therefore the vesicle gets displayed at the cell surface often enough that it can stick to a melanophore as its carrier macrophage wanders along.
When doing the research, was there a particularly exciting result or eureka moment that has stayed with you?
DSE I do not have good explanation for it, but I had very strong gut feeling that there are something going on between macrophages and airinemes. So of course, my most exciting moment was when I saw the macrophages interacting with extending airinemes.
Xanthoblast airinemes. Eom and Parichy, 2017.
And what about the flipside: any moments of frustration or despair?
DSE Our original plan was to add additional mechanisms of airineme vesicle-macrophage interactions beside phosphatidylserine, as we have several additional candidates. We ran into technical problems for this first paper but are now working on it again.
What next for you following this work?
DSE We have many questions about airineme-macrophage interactions, for example, what other signalling molecules carried in the airineme vesicles and how macrophages know what the targets are. I’m very excited about using our new super-resolution microscopes – they will open up many new possibilities.
“Our new super-resolution microscopes will open up many new possibilities.”
And where will this discovery take the Parichy lab?
DP Of course we’ll continue to pursue the mechanisms underlying this signalling relay in zebrafish pigment pattern formation. And we’d really like to know how these and other interactions have evolved to generate the very different patterns we see in Danio and beyond. But you know, a lot depends on the interests of the grads and postdocs who come to my lab. I just try to foster an intellectual environment and provide the resources to let people explore and go where the science leads. When we decided eight years ago to invest in live imaging we didn’t know what we’d find and we certainly didn’t expect this. So I’d be hesitant to make firm predictions.
Finally – what do you get up to outside of the lab when you are not playing with fish?
DSE One of my favourite things to do is visiting local breweries. My wife and I have a Saturday routine of having a lunch at a brewery, and then heading to the lab.
DP Legos. I like to spend as much time as I can with my four year old and my wife. So we do a lot of legos, trains and construction. Helicopters are big, too.
Dae Seok Eom & David M. Parichy. A macrophage relay for long-distance signaling during postembryonic tissue remodeling. Science, 355(6331): 1317-1320
Browse the People behind the Papers archive here


 (1 votes)
(1 votes)