The people behind the papers – Giri Dahal, Sarala Pradhan & Emily Bates
Posted by the Node Interviews, on 2 August 2017
Ion channels are famous for their roles in neurons and muscles, but the spectrum of phenotypes seen in ion channel mutants indicate a diversity of roles in development; the underlying mechanisms, however, have remained opaque. This week we feature a paper published in the latest issue of Development that reveals a link between potassium channels and morphogen signalling during epithelial morphogenesis. Co-first-authors Giri Dahal and Sarala Pradhan, and their PI Emily Bates of the University of Colorado Denver, told us more.

Emily, can you give us your scientific biography and the main questions your lab is trying to answer?
EB My long term goal as a high school student was to understand the mechanism behind migraine headaches and I thought that genetics would be the best tool to do that. The ACCESS program for women in science at the University of Utah placed me in Dr. Anthea Letsou’s lab and that was a great head start for my career. Dr. Letsou introduced the powerful tools available in Drosophila genetics and BMP/Dpp signalling. I stayed in her lab for four years working on defining the role of Punt, a type II Dpp receptor. I then went on to Harvard Medical School for my PhD in Dr. Anne Hart’s laboratory to focus on genetic neurological disease. I took classes about ion channels at Harvard, getting me closer to my goal. Then I joined Dr. Louis Ptacek’s lab at UCSF to study a genetic link to migraine. Dr. Ptacek’s lab focuses on episodic disorders including migraine and Anderson Tawil Syndome, caused by mutations in Kir2.1, a potassium channel. When I saw that in addition to periodic paralysis, mutations in Kir2.1 cause birth defects in people and that the phenotypes in model organisms look like the BMP signalling mutants, I became intrigued. That brought me to one of the central questions my lab is trying to answer: How do ion channels and electrical activity influence developmental signalling? We also have project that are trying to figure out how properties of the cytoskeleton influence neurodevelopment and neurodegeneration.
Giri and Sarala – how did you come to join Emily’s lab?
GD I came to the USA in the fall of 2008 to pursue graduate study at Brigham Young University. Emily was an Assistant Professor there. She had a positive outlook towards life and her views in science were inspiring. She wanted to pursue two projects, both were interesting but something about this project caught my attention. I was intrigued to find that we do not know why Kir channels cause dysmorphic features in Andersen-Tawil Syndrome even though scientists have been studying the channels for a long time. I liked both her personality and the project so decided to join her lab. I went with her to Colorado when she started her position at the University of Colorado.
SP Two ‘D’s attracted me, Drosophila and Denver, but certainly the research that was happening in the lab was key in my decision.
Can you give us key results of the paper in a paragraph?
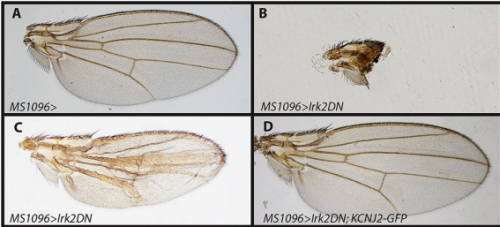
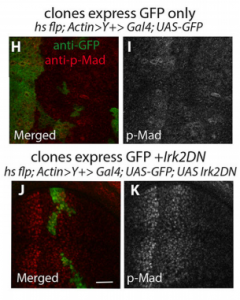
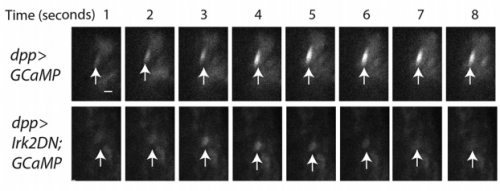
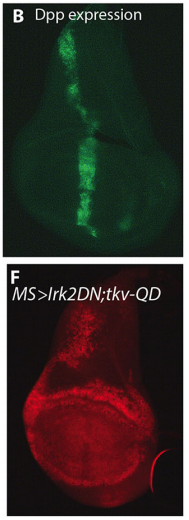
EB/GD/SP We knew that Irk channels played a role in Dpp signalling from our first paper that was also published in Development: Dahal et al 2012. The goal of our work since then has been to determine the molecular mechanism. We found that human Kir2.1 could substitute for fly Irk channels, showing that the developmental role of Irk/Kir channels is conserved between flies and humans. We found that Irk channels are important in the cells that produce Dpp and not in the cells that receive the signal. Inhibition of Irk channels in the Dpp-producing cells does not decrease the amount of Dpp that is made, but it changes the temporal dynamics of Dpp release. Irk channels modulate intracellular calcium levels to influence releases of insulin from pancreatic beta cells and neurotransmitter from neurons, so we hypothesized that Irk channels could regulate Dpp release via the same mechanism. Therefore, we looked for calcium activity in the wing disc. We found native transient increases in intracellular calcium that were altered by inhibition of Irk channels. Lastly, we found that depolarizing cells by applying a potassium solution to the extracellular bath caused transient increases in intracellular calcium and Dpp release.
Why might the timing, and not just the bulk amount, of Dpp release be important to development?
EB/GD/SP The timing of exposure to Transforming Growth Factor-beta (TGF- β) impacts the transcriptional response in tissue culture. For example pulsed exposure to TGF-B had a much greater transcriptional response than constant exposure to the same concentration of the ligand (See Sorre and Warmflash 2014). Dpp belongs to the TGF- β superfamily. Perhaps exposure to these ligands must be pulsed to signal efficiently. There are other examples of this in biology such as insulin signalling affecting metabolism and neurotransmitter affecting neurons. Similarly, the timing of notch signalling in somitogenesis is essential for its effect on the development of somites.
How similar is Dpp release from wing cells to neurotransmitter release from synapses?
EB/GD/SP Irk channels help to maintain resting membrane potential in neurons and therefore help to determine when a neuron will “fire” or undergo an action potential, increase intracellular calcium, and release neurotransmitter. In the context of the wing disc, we have shown that Irk channels influence calcium activity and Dpp release. We do not yet know if the release machinery is the same for neurotransmitter release and Dpp release.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
GD I would rather like to say that I have experienced surprising moments instead of a eureka because when it happens my first inclination is to think it is a fluke, which is true most of the time. By the time you confirm the result with enough samples and rule out other possible explanations the prospective eureka moment disappeared long ago because there is no element of surprise. I had small eureka moments with each new channel tested during the rescue experiment.
SP Yes, there were several. The most memorable were being able to see the regulation of calcium transients in the wing disc cells by Irk and the evoked Dpp release in wing disc cells by depolarizing with high extracellular potassium. These kinds of experiments that can be visually examined leaves one with a long lasting excitement.
And what about the flipside: any moments of frustration or despair?
GD Initially, we tried electrophysical approach to study mechanism of the Irk channels I took a course on the topic, and spent months to learn the technique. However, when I tried to express the fly Irk channels in Xenopus egg to measure current these channels did not express well so we abandoned this approach
SP There were many moments of frustration, despair, bruises and cuts. Thankfully these are like labor pains, once the child is born; they seem to fade away and you are ready to start again.
What are your career plans following this work?
GD For my postdoctoral training, I joined Dr. Howard Rockman lab at Duke University, where I worked to develop exosome-mediated drug delivery system to modulate cardiac function. Gradually, my interest shifted towards the downstream process of drug development. I decided to move out from academia. At present, I work in a company as a part of clinical operation team to conduct oncology trials.
SP I have always been ambitious to explore career challenges. As a child in Nepal I envisioned myself in white lab coat being a scientist somewhere. I first set foot in science with a degree in microbiology and taught in a brand new medical school in Nepal. It was a good job but I did not find myself challenged enough, so over-ambitiously I set my foot in USA to pursue my PhD. The day I graduated I felt overwhelmingly happy. Deep in my heart I was bothered with a question….that now I have achieved my life’s biggest goal….. what next? It may not be easy to live rest of my life without a goal. So I thought about it and set myself another goal and this time I did not want to be over ambitious. I just wanted to carry the responsibility and legacy of this degree with dignity for rest of my life. I feel that I have achieved this to some extent with this post doc paper in ‘Development’, and with being a biology faculty in college. So with this, I will continue to pursue a career in science. This may take different directions depending on the opportunities that open up and the different paths of life. I am open to new experiences and challenges that will build on my foundation in science and research.
And what next for the Bates lab?
EB We are now identifying other ion channels and ion channel associated proteins that are important for wing morphology and trying to determine if the SNARE complex is important for release of Dpp. In addition, we have evidence that Kir/Irk channels influence BMP signalling in mammals for craniofacial and limb development. To understand how Kir functions in mammals, we are extending our studies into mice.
Finally, what do you like to do when you are not in the lab?
EB My husband and I have a new baby girl, so currently my favourite thing to do outside the lab is play with her and watch her play with her Dad. I also enjoy family hikes and anything outdoors.
GD I live with my wife and two children. I spend most of our time outside of the lab taking care of our children.
SP Physically and mentally travel to Nepal and elsewhere if I can afford it in terms of time and money. But if you are talking about day-to-day activities, I enjoy playing with my son, being in nature, specially around water. I find the sound of water peaceful. And cooking delightful and delicious food at home.
, ,
This is the 25th episode of our People Behind the Papers series – browse the archive here.


 (5 votes)
(5 votes)