The people behind the papers – Simon Lane & Keith Jones
Posted by the Node Interviews, on 4 October 2017
Checkpoints ensure that mouse oocytes with DNA damage arrest in meiosis I, preventing non-viable embryo formation, however the mechanisms which activate this checkpoint have so far eluded researchers. This week we feature a paper published in the latest issue of Development that reveals that the unique ability of mouse oocytes to sense DNA damage by rapid kinetochore checkpoint activation. First author Simon Lane and his PI Keith Jones of the University of Southampton, told us more.

Keith, please can you give us your scientific biography and the main questions your lab is trying to answer?
KJ I am Head of Biological Sciences, and Chair of Cell Biology at the University of Southampton. In the mid 1990s I worked at the Medical Research Council Experimental Embryology & Teratology Unit in London, examining the way in which sperm-driven changes in intracellular calcium at fertilization were regulated and how they affected embryo quality, helping to establish a link between early events of fertilization and long term embryo quality.
Between 1998 and 2008 I held an academic position in the Institute for Cell and Molecular Biosciences at the University of Newcastle-upon Tyne, UK. My lab has helped develop the use of Fluorescent Proteins to study the process of meiosis in real-time. This approach led to recent developments in the understanding of how the meiotic divisions are regulated. In 2008, until 2012, I moved to University of Newcastle, Australia, where Simon joined me as a PhD student. In 2012 I moved back to the UK and Simon followed me, then as a postdoc having successfully defended his thesis.
My lab is focused on understanding how the oocyte makes the transition in meiosis from a mature egg.
Simon, how did you come to join the Jones lab?
SL After receiving an interesting lecture on fertilisation in ascidian eggs I applied for a summer placement in the cell biology labs in Newcastle University. There I met Keith who was about to move his lab to Australia and was recruiting PhD students. I didn’t hesitate at the opportunity to do a PhD in a subject that was very interesting and in such an exciting part of the world!
Can you give us a brief summary of why you decided to ask the questions in your paper and the previous research that led you to this story?
KJ Along with the John Carroll lab (then UCL, now Monash) we made the original discovery of the ability of oocytes to arrest in meiosis I a few years ago.
The current work is an extension of that initial study. The first study was all about reporting the phenomenon. The present one, published in Development, is a more detailed investigation of the mechanism by which arrest is achieved.
Can you give us the key results of the paper in a paragraph?
KJ In a nutshell it shows that oocytes have a profound ability to arrest in meiosis I in response to DNA damage, and that the mechanism by which this achieved is unusual. It’s a process that involves the kinetochore, rather than the sites of DNA damage, and it doesn’t involve the usual DDR kinases ATM and ATR. We also show that the response is specific to the first meiotic division, as it is absent in mature eggs.
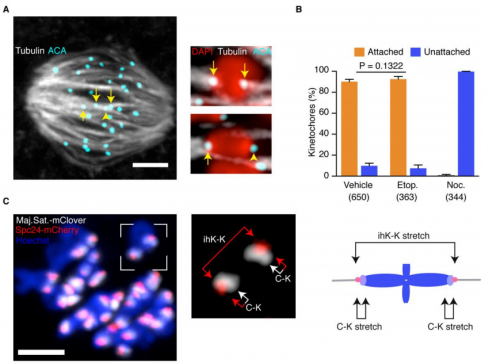
You suggest three models for MI oocyte sensitivity to DNA damage, how might you proceed to test your preferred model?
KJ The paper shows the response specific to the first meiotic division. We are therefore pursuing the role of various proteins known to play specific functions during the first but not the second meiotic division.
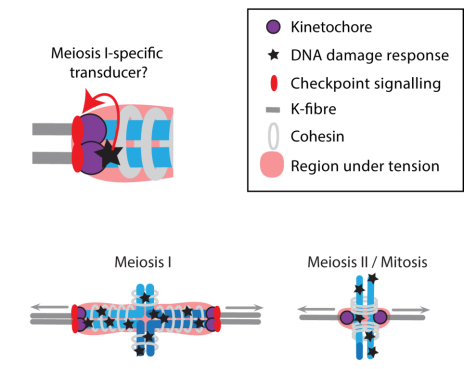
In the paper you talk about potential implications for human oocytes: how well do you think the mouse model translates and do you have any plans to test this research in human cells?
KJ We already know that follicular fluid collected from the ovaries of women who have endometriosis is able to cause arrest of mouse oocytes during in vitro maturation. The mechanism we believe is that ROS levels are higher in endometriosis, a phenomenon associated with inflammation, and the increased free radicals have the ability to damage DNA.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
SL I like the feeling when you are working late to complete an experiment but then you see something new and interesting and you think to yourself, I might just be the first person who has ever seen this. The experiment where the Mad1 response to DNA damage is completely different between oocytes and eggs was like that.
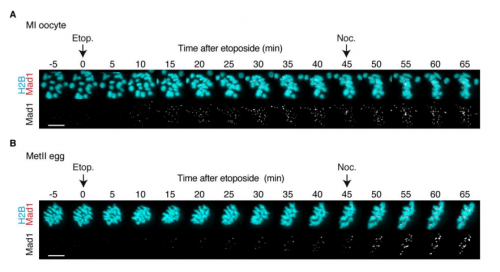
And on the flipside: any moments of frustration or despair?
SL There are many of these moments (more frustration than despair), it’s a part of the process I guess. So many things have to come together at once to get each experiment working so it keeps you constantly on your toes!
What are your career plans following this work?
SL I’m currently in the process of applying for fellowships.
And what is next for the Jones lab?
KJ I’d really like to figure out how the kinetochore function in meiosis. This seems a little dull at first, yet the structure of the chromosomes and how the segregate in meiosis I are unique, with co-segregation of sister kinetochores happening only in this division. Understanding how this co-segregation is achieved and how the meiotic spindle microtubules interact with the fused sister kinetochores are probably the most fundamental unknowns in meiosis.
Finally, what do you two like to do when you are not in the lab?
KJ My partner likes to tell me that my work is my only hobby! I think, although am not certain, this is a windup. I enjoy walking, good wines (I have been really surprised at the excellent sparkling wines made in Hampshire- next to Southampton), cooking, and BBC4 podcasts-take these in any combination. My work takes me round the world so I consequently do enjoy travel.
SL I keep fit with boot-camp style training and also like to experiment with 3D printing and electronics projects.
Simon I. Lane, Stephanie L. Morgan, Tianyu Wu, Josie K. Collins, Julie A. Merriman, Elias Ellnati, James M. Turner and Keith T. Jones. 2017. DNA damage induces a kinetochore-based ATM/ATR-independent SAC arrest unique to the first meiotic division in mouse oocytes. Development. Volume 144, Issue 19, p3475-3486.
This is #29 in our interview series. Browse the archive here.


 (1 votes)
(1 votes)