The people behind the papers – Ding Li and Jianbo Wang
Posted by the Node Interviews, on 29 November 2019
This interview, the 72nd in our series, was recently published in Development.
Heart development in mammals is a beautifully complex process. Patterning, proliferation and differentiation are all coordinated with cell movements and tissue morphogenesis (for instance elongation, fusion, folding, looping). However, our knowledge of the molecular regulators of heart development currently outstrips what we know about the morphogenetic processes that create the functional structure. A new paper in Development seeks to understand tissue-level morphogenesis – and its molecular control – in the mouse secondary heart field. We caught up with first author Ding Li and his postdoctoral advisor Jianbo Wang, Associate Professor at the University of Alabama at Birmingham, to find out more about the paper.

Jianbo, can you give us your scientific biography and the questions your lab is trying to answer?
JW I have always been fascinated by developmental biology, and how limited sets of highly conserved genetic circuits can be used to create hugely diverse structures and organs in different organisms. I did my PhD with Terry Magnuson, investigating epigenetic regulation in embryonic and extra-embryonic development in the mouse. For my postdoctoral training, I studied mouse dishevelled genes during embryogenesis with Tony Wynshaw-Boris. Dishevelled is a multi-functional, modular protein that is crucial for both canonical and non-canonical Wnt/planar cell polarity (PCP) signalling in flies and frogs. To functionally dissect dishevelled genes in the mouse, I created domain-deletion and point mutations in Dvl2 to specifically block either pathway. It turns out that most of the defects in Dvl2 mutants, from neural tube closure to inner ear and heart development, arise from disruption of PCP signalling. After starting my own lab, I have continued to use the mouse as a model to explore the role of PCP-mediated morphogenesis in different contexts of mammalian development. In addition, we are trying to understand the impact of PCP gene variants on human biology and congenital defects. Finally, in collaboration with my colleague Chenbei Chang, we are also using Xenopus to decipher the mechanisms and logics of PCP during tissue morphogenesis.
Ding, how did you come to work in the Wang lab, and what drives your research today?
DL Back in 2012, I was searching for a postdoc training opportunity in America. Jianbo’s ad caught my attention because I was interested in cardiovascular biology and heart development research. I had a nice interview with Jianbo on the phone, came to his lab in June 2012, and started a seven-year-long journey. I recently started a job in a clinical genomics laboratory, overseeing sequencing of human patient samples. I still however pay attention to the latest developments and advances in the field of cardiovascular development, an interest planted deep inside me.
Why do you think knowledge of heart morphogenesis has lagged behind knowledge of the signalling and transcriptional networks involved in its patterning?
DL & JW This probably has multiple reasons. Historically, our fundamental understanding of how the heart forms is largely from studies of key transcription factors and signalling pathways, so naturally there are more labs and people working on them. This trend has been further fuelled by the rapid advance in genomic technology, which has made it feasible to delineate molecularly how signalling crosstalk acts upon epigenetic and transcriptional hierarchies for cardiac specification and differentiation. The knowledge from these studies holds tremendous potential for translational medicine, such as stem cell-based regenerative approaches for cardiac repair. These studies are exciting and significant. They have been, and will continue to be, one of the main driving forces of the field.
Studies of morphogenesis, on the other hand, require greater attention to biology at the cellular and tissue levels. They are time consuming and labour intensive to carry out, the data tend to be noisier, and the results are more likely to be regarded as ‘descriptive and correlative’ rather than ‘mechanistic and causal’. So there is probably less impetus for these studies. But sometimes detailed descriptions at the cellular and tissue levels are important because they inform us about things that molecular and genomic studies cannot, such as spatial organization and the temporal order of events underlying heart development.
Additionally, compared with other fields, studying heart morphogenesis is uniquely challenging because the rapid beating makes live imaging of the heart and its surrounding cardiac progenitor field quite difficult. Some new technologies, such as light-sheet microscopy and high-resolution episcopic microscopy, will help to overcome the technical hurdles for morphogenesis studies, whereas other technologies, such as single cell RNAseq and spatial genomics, may help to bridge the gap between our knowledge of heart patterning and morphogenesis.
Can you give us the key results of the paper in a paragraph?
DL & JW Our studies first reveal that in the mouse, the secondary heart field population (SHF) in the splanchnic mesoderm (SpM-SHF) normally grows in a polarized fashion to preferentially elongate anteroposteriorly. Loss of Wnt5a, however, leads to isotropic expansion of the SpM-SHF. We provide evidence that Wnt5a may act through PCP effector and formin protein Daam1 to form horizontally oriented actomyosin cables in the medial SpM-SHF, thereby generating the mechanical force to constrict SHF widening and promote its lengthening. Genetic labelling and tracing reveal that the Wnt5a lineage is a unique SHF subpopulation specified as early as embryonic day (E)7.5, and undergoes bi-directional deployment towards both the arterial and venous poles to contribute specifically to the pulmonary trunk and atrial septum, respectively. In Wnt5a null mutants, the Wnt5a lineage fails to extend into the arterial and venous poles, causing both outflow tract and atrial septation defects, both of which can be rescued by an activated form of Daam1. Interestingly, the Wnt5a lineage in the SHF also contributes to the pulmonary mesenchyme and proper morphogenesis of the airway, suggesting the intriguing idea that during the evolution of tetrapods for terrestrial life, Wnt5a/PCP was recruited by the cardiopulmonary progenitors to orchestrate morphogenesis of both the pulmonary airway and cardiac septation necessary for pulmonary circulation.
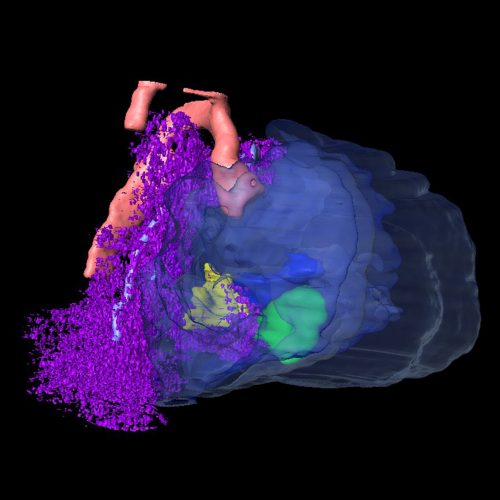
Is Wnt5a acting locally or at a distance in the SHF?
DL & JW This is a question that we debated a lot in the lab. The conventional wisdom in the Wnt field seems to be that the Wnt ligand does not travel very far, probably less than ten cell diameters away from the source. Our anti-Wnt5a antibody staining also shows a very restricted distribution in the caudal SHF, largely overlapping with the domain of Wnt5a mRNA expression. But we need to be cautious about the sensitivity of antibody staining. The fact that the SHF deployment defect in Wnt5a null mutants is not restricted to the Wnt5a lineage per se raises the possibility that Wnt5a may signal at some distance. In the limb, we have very strong genetic evidence that Wnt5a produced at a defined location can signal hundreds of microns away. The action of Wnt5a could be tissue specific though, so to address this question, we will need to tag Wnt5a and visualize its distribution.
Does your data have any relevance to the idea of cardiopulmonary progenitors?
DL & JW The idea of ‘cardiopulmonary progenitors’ was initially proposed by Ed Morrisey’s group to explain co-development of the heart and lung during the evolutionary adaptation to terrestrial life. They had found that the posterior SHF contains multipotent progenitors contributing to both the venous pole of the heart and pulmonary mesoderm. Our Wnt5a lineage-tracing data support this idea, and further demonstrate that the cardiopulmonary progenitors may also contribute to pulmonary trunk at the arterial pole, in addition to the dorsal mesenchymal protrusion (DMP) at the venous pole. Formation of the pulmonary trunk and DMP-mediated septation of the atria are both necessary to fully separate pulmonary from systemic circulation.
Recent studies from Ivan Moskowitz’s group further demonstrated that expression of Tbx5 in cardiopulmonary progenitors initiates a signalling cascade to specify pulmonary fate in the adjacent endoderm. We found that deleting Wnt5a using SHF-specific Mef2cCre causes not only cardiac septation but also airway morphogenesis defects. Therefore, we speculate that in addition to adopting the Tbx5 transcription network for pulmonary fate specification, the cardiopulmonary progenitors may have also recruited Wnt5a/PCP as a genetic circuit to orchestrate morphogenesis of the heart and lung during their co-evolution in tetrapods.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
DL I would say the most exciting moment during this project was when I finished the first 3D reconstruction of E10.5 wild-type and Wnt5a null mutant embryos. For the first time I was able to turn around and really see the whole heart in relation to the SHF on the computer display, not just a series of individual sections. That feeling was indescribable and the memory will definitely stay with me forever.
That feeling was indescribable and the memory will definitely stay with me forever
And what about the flipside: any moments of frustration or despair?
DL I have never had a project completely free of frustration, so of course this project was no exception. Here and there, we had big or small troubles with techniques, but somehow found ways to overcome and move on. The biggest frustration came from the clonal analysis. We tried to genetically label a few Wnt5a lineage cells by using our Wnt5aCreER mouse line and R26R-confetti (Brainbow) mouse line, and to test whether their deployment to the arterial and venous poles of the heart would change over time. However, the labelling efficiency of Wnt5aCreER with the confetti reporter was extremely low and we could not get any interpretable result after many tries. Finally, we gave up and had to submit the manuscript without this piece of data.
So what next for you after this paper?
DL After finishing the revision of this manuscript, I accepted a job offer from a clinical genomics laboratory, and started a career path in medical genetics and diagnosis. Although not dealing with embryos anymore, I do benefit a lot at work from my past life in research.
Where will this work take the Wang lab?
JW There are several things that we would like to pursue. First, we want to examine further the role of Wnt5a and PCP signalling in the co-morphogenesis of the heart and lungs. Second, we want to define further the action of Wnt5a by determining its signalling range, and whether it functions in a permissive or instructive fashion. Finally, we want to understand how the human WNT5A point mutations perturb its function during development and result in congenital birth defects.
Finally, let’s move outside the lab – what do you like to do in your spare time in Birmingham?
DL I like hiking. My favourite trail is on the top of the Red Mountain, where Birmingham’s famous Vulcan statue stands. Walking along the trail and overlooking downtown Birmingham is just really relaxing.
JW I was surprised to find how liveable Birmingham was when I first moved here from San Diego, and discovered a large number of fantastic restaurants of different cuisines. When my kids were younger, I hiked and mountain biked a lot with them in the nearby mountains, and practiced karate with them. Now I tend to do activities that are more relaxing, like yoga and golf. The Robert Trent Jones golf trail is a series of world-class golf courses built throughout the state of Alabama, and there are two of them within a 10 minute drive from my house.


 (No Ratings Yet)
(No Ratings Yet)