Organelle Assembly in Vivo: The Love-Hate Relationship of Thermodynamic and Active Processes
Posted by Hanieh Falahati, on 6 March 2017
Comment on ”Independent active and thermodynamic processes govern nucleolus assembly in vivo”, Proceedings of the National Academy of Sciences, 114 (6), 1335-1340, (2017).
Hanieh Falahati, Lewis–Sigler Institute for Integrative Genomics, Princeton University.
Eric Wieschaus, Howard Hughes Medical Institute, Department of Molecular Biology, Princeton University.
The whole universe is moving toward disorder; this is the second law of thermodynamics in simple terms. Yet, living organisms have found a way to keep themselves organized by spending energy. Cells need this organization in order to provide specialized microenvironments for different cellular functions. Two types of intracellular organizations can be distinguished in biological systems: The first type is membrane-bound organelles such as ER, or lysosome. The composition of these organelles is maintain by spending energy and active transport of molecules across a membrane. The second type of intracellular organization comes from membrane-less organelles such as nucleoli, histone locus bodies, and stress granules that are high-concentration assemblies of different proteins and RNAs. A puzzling question is that without a membrane, how are cells able to form and maintain these organelles.
A historical perspective
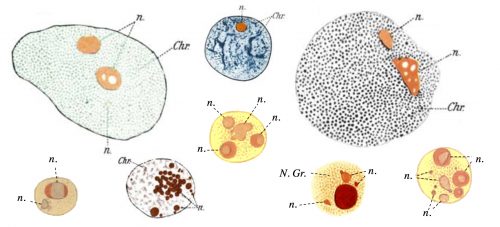
The question of how membrane-less organelles form immediately captured my attention the first time I heard about it in an introductory meeting with my doctoral advisor, Prof. Eric Wieschaus. Of course this question has fascinated many scientists since the initial observation of the nucleolus, the quintessential membrane-less organelle, in the 18th century. In 1898, Montgomery performed a very comprehensive study of the nucleoli of different cell types, hand-drawn in 346 figures (a sample is shown in Fig. 1), and concluded that the nucleolus forms when a substance from the cytoplasm enters the nucleus and is deposited there “in the form of masses of varying dimensions, which may be either globular or irregular in shape, according as they are fluid or viscid in consistency”. He further describes that nucleoli form via “coalescence of numerous small portions of nucleolar substance”, consistent with its fluidity (Montgomery 1898). However, this model faded away with the advancement in cell biology and genetics which showed that the nucleolus forms around ribosomal DNA repeats and is the site of active transcription and processing of ribosomal RNA, and ribosomal biogenesis. By the end of the 20th century, nucleolus was commonly thought to form actively, in the process of making ribosomes, until a number of influential works from Hyman and Rosen groups redirected the focus to the liquid nature of the membrane-less organelles (Brangwynne et al. 2009; Li et al. 2012). Based on this liquid property, it was proposed that formation of membrane-less organelles is a spontaneous liquid-liquid phase separation (LLPS), similar to the separation of oil from water.
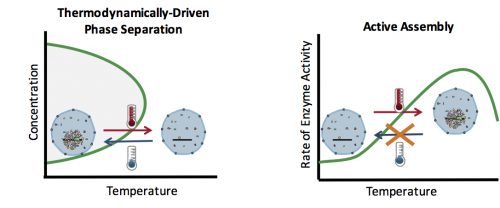
By the time I joined the Wieschaus lab at Princeton, two main hypotheses existed for the formation of membrane-less organelles: 1. The LLPS model, in which the components of these organelles assemble spontaneously in a thermodynamically-driven process due to their favorable intermolecular interactions, and form a liquid phase; 2. The active assembly model which suggests that these organelles form as a result of an active process, which happens inside the cells or inside the organelles. Such active processes are reactions carried out by enzymes that couple these reactions to an energy source such as ATP. The evidence for the LLPS model comes from experiments showing that many of the proteins which localize to the membrane-less organelles have the physical properties to phase separate in vitro. However, in most cases the phase separation is only observed at concentrations much higher than cellular levels. Therefore, a pressing question is whether the physical properties of these components would also drive their spontaneous assembly in vivo, or active processes are responsible for their congregation. Accordingly, we decided to develop an in vivo test that would allow us to distinguish between the LLPS and the active mechanism, and apply it to study nucleolus assembly as a model for the formation of membrane-less organelles.
Working out a path – How to test the phase separation model in vivo?
Developing such an in vivo test was not trivial, since compared to in vitro systems, living cells are far more limited in the ways they can be manipulated. But considering the expertise of my advisor, Eric Wieschaus, in manipulating Drosophila embryos, his lab was the best place to do this type of research. It was not the only reason for us to study this question in fly embryos: Development in Drosophila starts without a nucleolus, and the nucleolus forms at a particular developmental stage (Video 1). This allows us to manipulate the system and study its effect on the nucleolus assembly. In addition, nucleolus forms during the 13 synchronous divisions at the beginning of development, therefore at each time-point there are multiple nuclei at the exact same developmental stage, which helps with statistics. Finally, during these 13 division the nuclei share the cytoplasm, which means that all the nuclei have access to the same concentration of most molecules. These features make fly embryos an ideal system for studying nucleolus assembly.
Video 1. Nuclei of D. melanogaster embryos start development without a nucleolus, and form the nucleolus (bright foci) for the first time at nuclear cycle 13.
How can you test whether the formation of an organelle in vivo is a thermodynamically driven LLPS, or an active assembly process? In general, LLPS processes are affected by two factors: concentration and temperature (Fig. 2). Increasing the concentration results in an increase in the size of assemblies: The more oil you add to the water, the bigger the oil droplets. However, active processes are also affected similarly with concentration: The higher the concentration of the reactants, the more products (assemblies) are formed. Therefore, changes in the concentration cannot distinguish between an LLPS and an active assembly. On the contrary, temperature often has a differential effect on these two processes: In general, lowering the temperature causes more condensation and enhances phase separation, but slows down active enzymatic reactions. This means that by quantifying the effect of various temperatures on the assembly formation, one can distinguish between an LLPS and an active assembly model.
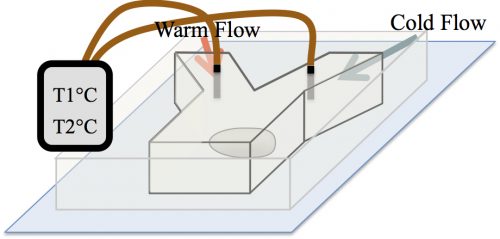
At the time, our strategy to examine the effect of temperature on the formation of assemblies was received with skepticism among the community. The main concern was whether in the temperature range tolerable by biological systems, an effect would be detectable. A reassurance, however, was an exquisite work by Sarah Veatch, who showed that plasma membrane vesicles isolated from living cells exhibit phase transitions between 15 and 25°C (Veatch et al. 2008). Subsequently, we employed a microfluidic device that allowed us to control the temperature of a fly embryo between 6 and 31°C (Fig. 3). We used this microfluidic device to examine the effect of temperature on the first time formation of assemblies, and also to test the reversibility of assembly formation, for six different nucleolar proteins.
The unexpected result of two independent mechanisms
Much to our surprise, the results of our test showed that two independent mechanisms govern the assembly of different subsets of nucleolar proteins: While four of the studied proteins depict properties of LLPS, the two others are recruited actively. At lower temperatures, the phase separating proteins assemble at an earlier developmental time, and dissolve upon heating (Video 2). The latter is consistent with the reversibility of thermodynamic processes. On the contrary, the formation of active proteins is inhibited at low temperatures, and is irreversible, an exclusive property for active processes.
Video 2. The nuclei of intact fly embryos are subjected to temperature changes in the surrounding fluid. As the temperature is shifted from low to high, the phase separated proteins dissolve, as can be seen in the disappearance of the bright spots.
rDNA: Come together, right now, over me
Our unexpected result that two independent mechanisms govern nucleolus assembly in vivo raised a new question: How are these two different mechanisms coordinated to form a single organelle? In a previous paper, we had shown that transcription of rDNA dictates the spatiotemporal precision in the formation of assemblies by the phase separating proteins (Falahati et al. 2016). Interestingly, we were also able to show that rDNA is necessary for the recruitment of the two actively assembling proteins. This suggests that rDNA can function as a coordinator between the two independent mechanism. In addition, it highlights the fact that in vivo, even the assembly of phase separating proteins is regulated by active processes such as transcription.
We are very enthusiastic about the publication of this paper as it is a significant advancement in the field both from a methodological and mechanistic standpoint. We took an interdisciplinary approach and developed an invaluable tool for closing the gap between the current knowledge of the in vitro self-assembly and the formation of membrane-less organelles in vivo at its full complexity. From a mechanistic perspective, the large number of proteins studied here allowed us to unravel the unappreciated complexity in the formation of intracellular assemblies, as our results show the presence of at least two independent mechanisms for the recruitment of nucleolar proteins. We are confident that the method and the results introduced in this paper will set a framework to better understand how normal or pathological intracellular assemblies form.
References:
- Brangwynne, CP et al. 2009. “Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation.” Science 324(5935): 1729–32.
- Falahati, H., Pelham-Webb, B., Blythe, S., & Wieschaus, E. 2016. “Nucleation by rRNA Dictates the Precision of Nucleolus Assembly.” Current Biology 26(3): 277–85.
- Falahati, H., & Wieschaus, E. 2017. ”Independent active and thermodynamic processes govern nucleolus assembly in vivo.” Proceedings of the National Academy of Sciences, 114 (6), 1335-1340.
- Li, P., Banjade, S., Cheng, H.C., Kim, S., Chen, B., Guo, L., Llaguno, M., Hollingsworth, J.V., King, D.S., Banani, S.F. and Russo, P.S. 2012. “Phase Transitions in the Assembly of Multivalent Signalling Proteins.” Nature 483 VN-(7389): 336–40.
- Montgomery, Tho S. H. 1898. “Comparative Cytological Studies, with Especial Regard to the Morphology of the Nucleolus.” Journal of Morphology 15(2): 265–582.
- Veatch, S. L., Cicuta, P., Sengupta, P., Honerkamp-Smith, A., Holowka, D., & Baird, B. 2008. “Critical Fluctuations in Plasma Membrane Vesicles.” ACS Chemical Biology 3(5): 287–93.


 (2 votes)
(2 votes)