The people behind the papers – David Turner & Peter Baillie-Johnson
Posted by the Node Interviews, on 6 November 2017
Embryonic patterning is dependent on the establishment of the anteroposterior (AP) and dorsoventral axes early in development. In mammals this occurs by a breaking of symmetry in the epiblast, however the molecular mechanisms controlling this process are still not fully understood. This week we feature a paper published in the latest issue of Development that models these patterning events in gastruloids. Two authors: David Turner and Peter Baillie-Johnson from the Martinez-Arias group at the University of Cambridge told us more.

David and Peter, can you give us your scientific biographies and the main questions the lab is trying to answer?
DT Our lab is primarily concerned with understanding the early decisions involved during mammalian development. Specifically, how the early mouse embryo patterns itself and specifies the body axes. We mainly use mouse embryonic stem cells as a model system to study these early developmental processes both in normal monolayer culture conditions (2D) and also using our gastruloid model system in 3D (embryonic organoids).
DT For quite some time I’ve been interested in cell signalling in general and how cell fate is determined, an interest which was initially sparked during my Pharmacology honours degree in Liverpool. Following in this vein, I took up a PhD (also in Liverpool) with Prof. Mike White (who’s now in Manchester) where I used single-cell fluorescence imaging to investigate the dynamics of NF-kB in response to low doses of the cytokine TNFa.
PBJ I studied Natural Sciences as an undergraduate at Cambridge from 2010-2013, specialising in Genetics in my final year. I started my PhD in October 2013 and handed in the final copies of my thesis in October 2017. During my PhD, I worked on developing the gastruloid system and applying it to the origin of the mammalian spinal cord. Since April this year, I’ve been working as postdoctoral research assistant to Professor Jenny Nichols, who has helped me shift my focus to earlier stages of mouse development, namely gastrulation.
David and Peter, how did you both come to join the Martinez Arias lab?
DT Having finished my PhD in Liverpool, I saw a post-doc position open in Alfonso’s lab which was about using mouse embryonic stem cells as an in vitro model system to try and understand their properties and their differentiation potential. My background from my PhD was strongly in single-cell imaging and cell signalling so it was a perfect opportunity for me to pursue my interests.
PBJ I first joined Alfonso’s lab in 2012 as a summer student during my undergraduate course. At that time, the lab was more focused on the regulation of embryonic stem cell pluripotency and differentiation. I was really taken with this introduction to stem cell biology and felt warmly welcomed into the lab, so I applied to follow the work up in my final year research project. While awaiting the results of my finals, I ran into David and heard about the first gastruloid experiments, which naturally became the focus of my PhD.
Your paper addresses the question of axis establishment in the early mammalian embryo. What was known about the molecular control of polarity prior to your paper?
PBJ The textbook models of antero-posterior axis specification in the mouse describe an opposing arrangement of signals and their inhibitors emanating from the extra-embryonic tissues that surround the then radially-symmetric epiblast. These models describe how the signals in the future posterior are balanced in the future anterior by their corresponding secreted inhibitors. The cells of the epiblast are then restricted to undergo a localised EMT (i.e. the beginnings of the primitive streak) only in the future posterior region, while the anterior epiblast remains reserved for the anterior nervous system. The key feature of this model is that the asymmetry originates in the extraembryonic tissues, which then becomes conferred on the underlying epiblast. Following our study, it now seems as if this careful balance of signalling across the embryo might act to permit an intrinsic symmetry-breaking event in the future posterior, rather than actively instructing the process. It remains to be seen whether the lack of the anteriorly expressed inhibitors in the gastruloids fully explains the lack of anterior structures that we have observed.
Why did you use gastruloids and not an in vivo system for this project?
PBJ I think that the acquisition of antero-posterior polarity is a good example of a topic that has been well-described in the embryo through genetic experiments. I think the strength of the gastruloids as an experimental tool is in providing insights on development that would not be possible by looking at the embryo alone – in this case by looking at development in the absence of the extraembryonic tissues and the post-implantation mechanical microenvironment. This In vitro system also enables the possibility of identifying the sufficient components behind a genetic process by starting with a minimal set of interacting parts. This approach is quite different to genetic experiments, which are good at identifying the necessary components of developmental systems, but which can’t (easily) demonstrate sufficiency.
DT Echoing what Peter said, the gastruloids have a significant advantage over in vivo systems in that the developmental processes and events we’re interested in occur at a time in the embryo that’s difficult to access and manipulate experimentally. With our gastruloids, we’re able to ask very specific questions about subtle timings of signals in ways which are either very difficult or impossible to do in the embryo, such as pulses of signals at very precise temporal intervals. Compared with in vivo models, gastruloids are relatively inexpensive, easy to manipulate, and amenable to experimental perturbation. Also, because of this ability to recapitulate many of the early developmental processes (mentioned above), our system has the real potential to be used as a way to reduce or replace animals used for research in development, which is central to the aims of NC3Rs (National Centre for the Replacement, Refinement and Reduction of Animals in Research).
Can you give us the key results of the paper in a paragraph?
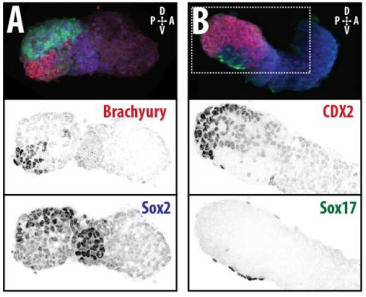
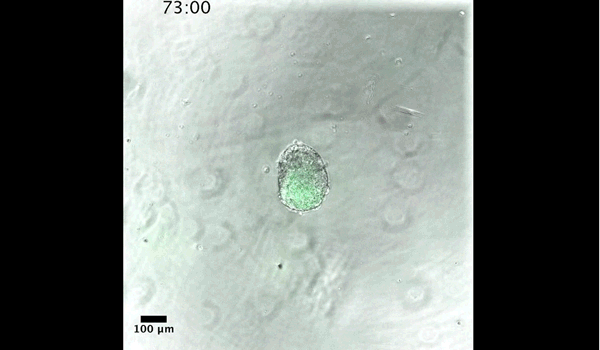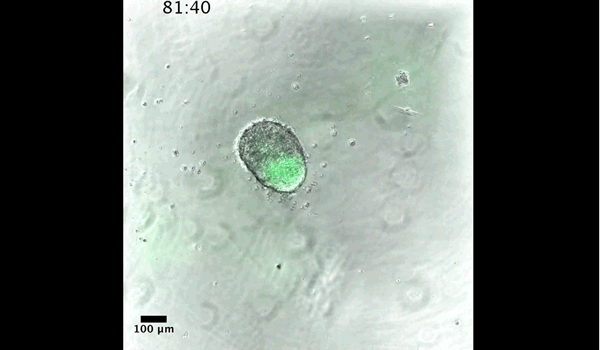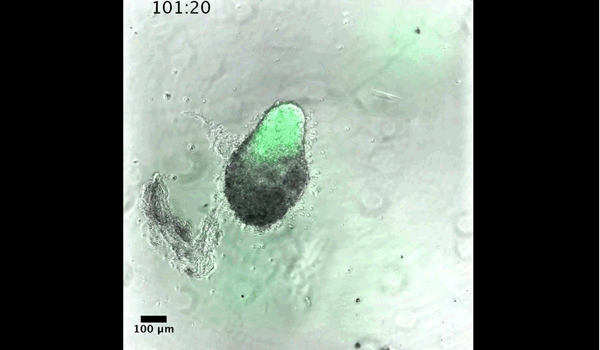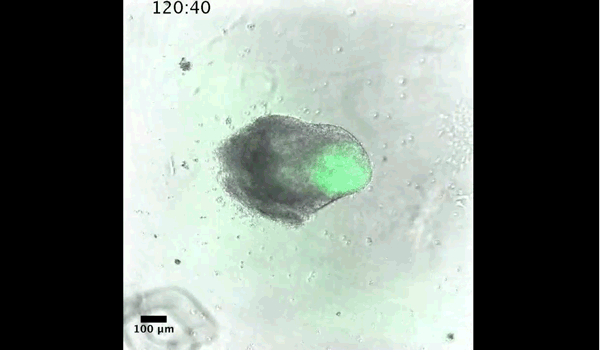
DT The main aim of the paper was to use gastruloids to study anteroposterior patterning. We found that gastruloids spontaneously break symmetry, polarise gene expression and undergo axial elongation in a robust and reproducible manner similar to the early embryo. One end of the gastruloid resembles the posterior region of the embryo, where Brachyury is up-regulated. Our quantitative analyses show that this is regulated by both Nodal and Wnt/b-Catenin signalling, and surprisingly with no detectable involvement of BMP signalling. Our most interesting finding is that the AP axis can form without any of the extraembryonic tissues which have been suggested to be important for this process. This lead us to hypothesise that the role of the extra-embryonic tissues in the embryo may not necessarily be to induce the AP axis, but to bias the intrinsic symmetry-breaking potential of the embryo.
To what extent does gastruloid patterning recapitulate embryonic development?
DT I think that the gastruloids can recapitulate many aspects of early post-implantation embryonic development in the mouse and at a similar timescale to the embryo. First and foremost is their ability to develop AP polarity with Brachyury expression at one end (which we have designated the posterior) and in the DV direction where Sox2 is directly opposite Sox17. Furthermore, they’re able to extrude cells from the extending, Brachyury-positive region, in a manner akin to gastrulation at the primitive streak (hence their name). Gastruloids do not, however, develop the pre-occipital tissues of the head and brain, so their antero-posterior axis probably only represents the post-occipital levels of the embryo. We think this is due to a lack of tissues that would protect this region from high levels of ‘posteriorising’ signals (such as the prechordal plate and anterior mesoderm). It remains to be seen exactly which axial levels are represented in these seemingly posterior tissues and what we need to do to expand this representation.
PBJ I think an interesting feature of gastruloid development is the time over which the events unfold, which in our standard cultures corresponds approximately to the five days after implantation. It’s striking how they always undergo the same progression of changes in gene expression and morphology in this time and this is, for me, a key reason for using them to investigate early developmental events such as gastrulation.
In addition to your own gastruloid research, other recent papers have described systems that aim to recapitulate early development in vitro. This has generated considerable media attention and has sometimes been described as ‘creating artificial life’. How do you feel about this description? More generally, do you think there are any ethical issues thrown up by research using so-called synthetic embryos?
PBJ I think that this is a sensitive issue that certainly demands careful ethical consideration – perhaps in a longer form than an interview. Although it’s a semantic point, I think that the description of “creating artificial life” is imprecise and unhelpful and I’d prefer to see “engineering developmental systems” used instead. I think that this emphasises that these systems are controlled approaches that can recreate features of embryonic development, but which might otherwise be limited in their developmental potential.
Many of the ethical considerations surrounding these experiments and ultimately the scope of the legislation that will regulate them hinge on how exactly we define an embryo. For example, if researchers constructed a set of tissues from specific cell types in the exact image of the early embryo, would we consider the two to be equivalent? Conversely, how would a structure that closely resembles a significant proportion of the embryonic body measure up to the embryo if the representation was not complete? Would this logic extend further to individual organs or organoids? I think that scientists and the public need to consider these issues if we are to determine how we will regulate this form of research alongside the existing framework for research with embryos (which may itself need to be revised). With a clearer definition in hand, it will be easier to approach the deeper questions of where to draw appropriate limits of research on mouse and human systems, whether engineered or otherwise. To paraphrase a commentary from Martin Pera et al.1, the extent to which researchers can recreate embryogenesis in vitro will come to define not only how experimentally useful these systems are, but also how much scrutiny they will attract.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
DT I think one of the best moments was late in 2013 when Alfonso and I were watching a time-lapse movie on the microscope that Susanne van den Brink had just finished imaging (the lead author on the first gastruloid paper). This is where we saw, for the first time, cells being extruded from the elongating region of the gastruloids and realised how important and useful this system was going to be. A second important moment experimentally was during the early stages of optimising the gastruloid protocol, when we were trying to improve the reproducibility within each plate and between plates of gastruloids. We found that the simple act of adding a second wash of PBS improved all the stages of gastruloid formation: the aggregation, the patterning and the elongation, so this felt like an important breakthrough.
PBJ There have been a couple of those precious moments when you’ve seen something that no-one else has before, which have been real highlights for me. I think I was lucky to have worked on such an exploratory project for my PhD as those moments have really stayed with me. In general, however, the work has progressed incrementally but I’ve been consistently surprised by the level of autonomy that the gastruloids show as we’ve started to look more closely at their development.
And what about the flipside: any moments of frustration or despair?
DT We found, after quite a few frustrating early attempts, that the initial culture conditions are essential to ensure good formation of gastruloids, i.e. low passage numbers, consistent splitting ratios, plating density. It took a little while for us to realise this and to ensure that our stock flasks are treated in a consistent manner.
PBJ There were definitely frustrating times in the early stages of my PhD, when we hadn’t identified the key variables that could determine whether the cultures would thrive or fail. We also had moments of doubt as to whether our observations would prove to be new and useful biological insights, but our confidence in using gastruloids as an experimental tool has grown as we’ve learned more about their development.
What are your career plans following this work?
DT I was recently awarded a David Sainsbury Fellowship from the NC3Rs to use the gastruloid system to study left-right asymmetry during mammalian development, and for the next three years I’ll be working pretty much solidly on that!
PBJ I’m currently working as a postdoctoral Research Assistant to Professor Jenny Nichols, who is helping me to cut my teeth on mouse embryology. By studying the gastrulating mouse embryo first hand, I’m trying to determine how closely the process of “gastrulation” in the gastruloids measures up to that in the embryo. By doing so, I hope to determine whether gastruloids could be used as an experimental tool to dissect this complicated phase in the life of the embryo. My work is closely aligned to that of a Cambridge-based consortium that is investigating gastrulation through single cell genomics, so I hope that my work with the embryo will provide a reference for their findings and that the gastruloid system might offer a complementary approach in the future.
And what next for the Martinez Arias lab?
DT There are quite a few avenues of our research at the moment. One is to get more of a handle on what drives the elongation in gastruloids, whether it is a mechanism based purely on convergent extension or rapid cell growth, and whether the signals suggested to be involved during the axial extension of the embryo work in a similar manner in gastruloids. We’re also interested in seeing whether the gastruloid system is applicable to later stages of development and what its limitations might be; our ongoing collaboration with our co-authors in Matthias Lutolf’s lab at EPFL is an important part of this research.
Finally, what do you two like to do when you are not in the lab?
DT As little as possible to be honest, since free weekends and evenings are quite hard to come by when stem cell work is involved! Any free time I have I like to spend with my wife and my two children. Otherwise, I spend plenty of time reading and am currently working my way through Stephen King’s Dark Tower novels, which I can certainly recommend.
PBJ I love to be outdoors, so try to get out walking, running or cycling at the weekends. I’m also a keen cook and enjoy testing out new recipes on my friends and family.
David A. Turner, Mehmet Girgin, Luz Alonso-Crisostomo, Vikas Trivedi, Peter Baillie-Johnson, Cherise R. Glodowski, Penelope C. Hayward, Jérôme Collignon, Carsten Gustavsen, Palle Serup, Benjamin Steventon, Matthias P. Lutolf, Alfonso Martinez Arias. 2017. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development. Volume 144, Issue 21, P3894-3906.
This is #31 in our interview series. Browse the archive here.
- Pera, M. F. et al. What if stem cells turn into embryos in a dish? Nat. Methods 12, 917–919 (2015).


 (2 votes)
(2 votes)