In the apple eye of evolution: Camera-type eye regeneration
Posted by Shefali Shefali, on 23 September 2025
Behind the paper stories from “A genetically tractable non-vertebrate system to study complete camera-type eye regeneration“
We are all stepping into a story where evolution, development, and regeneration converge in the eye of a snail.

Throughout their lives, organisms encounter injuries and stresses that threaten the integrity of their bodies and have evolved remarkable ways to restore lost or damaged tissues. This ability to replace body parts, which can range from reorganizing existing structures to generating entirely new ones—is known as regeneration.
Among many forms of regeneration, the ability to rebuild eyes is especially striking. Eyes are among the most intricate organs, requiring precise anatomical organization and highly ordered neural wiring to restore function. Across the animal kingdom, eyes vary widely, reflecting adaptation to different ecological demands. While regeneration of simpler structures, such as planarian pigmented eye cups, and partial regeneration of camera-type eyes in vertebrates has been described, the idea that complete adult camera-type eyes could regenerate has long seemed improbable. These highly specialized organs, capable of high-resolution vision, present unique challenges that extend beyond conventional models.
In a recent groundbreaking Nature Communications study, Alice Accorsi, Alejandro Sánchez Alvarado, and colleagues demonstrate that the apple snail, Pomacea canaliculata can completely regenerate its camera-type eyes. By coupling this discovery with CRISPR–Cas9 genome editing, they establish a new genetically tractable model to probe regeneration of complex sensory organs. Here are behind the scene stories from the corresponding authors – Dr. Alice Accorsi and Dr. Alejandro Sánchez Alvarado.
First we have behind the science stories from Dr Alice Accorsi !

Accorsi lab
Bluesky: @accorsi-alice.bsky.social
Image source : Joaquin Benitez, College of Biological-Sciences, UC Davis.
How did you first get introduced to apple snails, and what drew you to them? Tell us about your PhD work.
Throughout my career I have worked with several invertebrate species, such as snails, leeches and planarians. These apple snails are originally from South America, particularly Brazil and Argentina, but have now spread to parts of Asia, Europe, and North America, where they pose a serious threat to local ecosystems. The same traits that make them invasive, such as resilience, rapid growth and prolific reproduction, also make them easy to care for. And it turns out this also makes them excellent laboratory models. My PhD mentor, Dr. Enzo Ottaviani, once purchased some apple snails from a pet shop and had them in his office. It was during one of our meetings that we wondered if we could use them as another invertebrate in my research! During my graduate studies, I was interested in studying their immune system to understand what makes them so resilient and to explore ways to affect their survival without using environmentally harmful compounds. I was also intrigued by the possibility that their immune and nervous systems might communicate with each other, as we see in vertebrates. My research uncovered evidence of this crosstalk, offering a new evolutionary perspective on neuroimmune interactions.
What convinced you to keep working with snails in your research – even during post doc and now in your independent research program as a faculty? What led you to the Sánchez Alvarado lab?
This journey began with a conversation between Dr. Alejandro Sánchez Alvarado (Stowers Institute for Medical research, Kansas City, MO) and me at the Marine Biological Laboratory in Woods Hole, MA. I was still a graduate student at the time, studying the immune system of apple snails, while Alejandro’s laboratory was focused on regeneration in planarians. Although snails have been known for their regenerative abilities since the 1700s, no one had explored their biology using modern molecular tools. That conversation sparked my interest in applying these approaches to snails to see what we could uncover.
We already have several model systems that excel at regenerating different body parts, such as planarians, hydras, and axolotls. I began to wonder whether these snails could regenerate an organ that the others could not, making them unique and even more relevant to study. That is when I discovered that apple snails possess complex camera-type eyes, the same kind of eyes found in humans. This opened up a unique opportunity to explore regenerative biology in a new way, with potential implications for human health. That is what convinced me to continue working with snails, even as I transitioned into postdoctoral and now independent research.
How was your transition from Italy to US for postdoctoral work?
Moving abroad for my postdoctoral studies was a major life change. I left my family behind and immersed myself in a new culture and scientific environment. I moved from a small lab with limited resources where I was the most senior member to the Stowers Institute for Medical Research, a place with nearly unlimited possibilities and a large, diverse team of scientists, including many senior researchers.
Despite the challenges, I never regretted the move. I learned more than I ever imagined and had the chance to connect with scientists across the country and the world. The Technology Centers at Stowers supported my work and introduced me to techniques I had only read about before. I am deeply grateful for the preparation I received through the Italian educational system, which gave me the foundation to take this leap.
What was it like to take on eye regeneration in snails – a phenomenon that hadn’t really been studied in them before?
Taking on a project about complete eye regeneration in snails was both exciting and challenging. Since this phenomenon had not been studied before and this was a relatively novel model system, we had to start from scratch. We began by characterizing the morphology of apple snail eyes using microscopy and histological techniques to understand their structure and cellular composition. Then, we performed genomic and transcriptomic analyses to identify the genes involved in eye development and regeneration. Finally, we developed techniques to manipulate their genome to test gene function.
This multi-approach research allowed us to build a comprehensive picture of apple snail eye anatomy, gene expression and regeneration, laying the groundwork for deeper investigations into the molecular mechanisms behind this process.
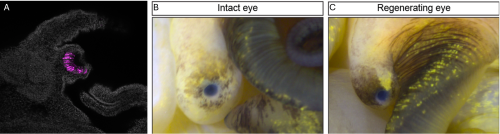
Your genomic analyses revealed genes shared between apple snails, humans, and Drosophila, particularly related to eye development and photoreceptor formation. What does this shared genetic toolkit tell us about the evolution of complex eyes across distant lineages?
Our molecular studies revealed that many genes are involved in forming both snail and human eyes, even though these eyes evolved independently. This suggests that, while there may be many ways to build an eye, the fundamental genetic building blocks are conserved between very different species (humans and snails). These findings have important implications for evolutionary biology. By comparing the development of camera-type eyes in snails, cephalopods, and humans we can shed light on how these complex structures evolved multiple times independently. This helps us identify both conserved mechanisms and evolutionary novelties across species.
Can you describe the moment you first saw a regenerated camera type eye?
Seeing the regenerated eye for the first time was exciting, but in that moment, I was not even close to fully grasp the importance of that one piece of data. It was later on, reading literature and looking through old papers and I started appreciating how this unconventional system could reveal something truly profound about regeneration. That realization was the real turning point that deepened my commitment to this research.
Your experiments showed eye regeneration unfolded in defined stages—wound healing, blastema formation, tissue emergence, and maturation. Did any of these phases surprise you ?
One of the most remarkable aspects of apple snail eye regeneration is how fast, precise, and reproducible it is. After complete eye removal, early signs of regrowth appear in less than two weeks, and a fully reconstructed eye, with all its components, is restored in under a month.
What surprised me most was the efficiency and consistency of this process. The speed at which regeneration unfolds, and the minimal variability between individuals, suggest a tightly regulated mechanism. Just as striking was the discovery that many of the genes active during regeneration are also involved in vertebrate eye development. This points to a shared genetic toolkit and opens exciting possibilities for comparative studies that could inform regenerative medicine.
Your pax6 studies reaffirmed its conserved function, do you think the role of pax 6 is binary ?
In our system, pax6 appears to play a binary role. When pax6 is knocked out, eye development is completely abolished. We did not observe any eye-related structures or any intermediate phenotypes, which underscores how essential this gene is. It is astonishing to see such a conserved function across species.
Do you plan to test the behavioral capabilities of regenerated eyes?
Absolutely. One of our main goals moving forward is to study the behavior and visual capabilities of apple snails. We are planning to collaborate with labs that specialize in behavioral neuroscience and vision to explore what snails can see in their environment and how well regenerated eyes can function.
What challenges did you face developing CRISPR lines?
Establishing stable CRISPR/Cas9 mutant lines in snails was a major technical challenge. A few steps were quite difficult. The first was collecting and injecting the zygotes, as they are very small! The next difficult step was ensuring their survival to adulthood after we removed them from the eggs. It took a lot of trial and error. Each step required patience and precision, but eventually, we developed a reliable workflow that allowed us to generate reproducible mutant phenotypes.
How do snails complement fly eye development in other model systems like Drosophila?
While Drosophila has been a powerhouse for studying eye development, its compound eyes are anatomically different from human eyes. Moreover, adult fruit flies do not regenerate their eyes after injury. Apple snails, on the other hand, have camera-type eyes, just like us, and can regenerate them completely.
This makes apple snails a powerful complementary model. Their regenerative abilities, combined with shared genetic pathways, offer a unique window into how complex organs can be rebuilt. Studying molecular pathways involved in eye formation and function across such diverse species helps us identify conserved mechanisms and evolutionary innovations, expanding our understanding of how regeneration evolved.
What was your most validating moment in this project?
The most emotional moment of this project was when I obtained pax6 homozygous mutants. I looked in the microscope without daring to hope for anything special. But after getting the embryos in focus, I saw that some of them did not develop eyes. That was the moment I knew CRISPR/Cas9 was working and the function of the gene pax6 was conserved in apple snails. It was incredibly validating and empowering. That was the moment when I truly felt I could start thinking about “the rest of my scientific career” as the leader of a lab using apple snails to study eye regeneration.
Can you share some challenging moments from the project. What were your ways to reset/unwind ?
One of the biggest challenges of this research was figuring out how to collect, inject and raise snail embryos to adults. This was a long, slow and meticulous process. I spent hours carefully observing embryos trying to pinpoint what was not working and letting the biology guide the adjustments. I for sure learnt patience and resilience through this process.
Outside the lab, I love to do yoga, listen to audiobooks and spend time with the people I love. These moments help me recharge and return to the lab with fresh energy.
Were there any quirky moments that shaped the trajectory of the study?
A quirky moment that shaped not just this study but of my entire career happened during graduate school. I was so excited about regeneration after attending the MBL Embryology Course in Woods Hole that I immediately wanted to test if the apple snails I was working on were able to survive injuries and regenerate. I got dissection scissors and… well, luckily for me and for them, they regenerated!
How did your team coordinate such a complex study?
At Stowers, I had incredible support from the Technology Centers, which helped optimize protocols, run experiments and maintain the snails. At UC Davis, we also have excellent core facilities for imaging and sequencing, but the members of my lab play a central role in all the work that we do. I encourage everybody on my team to learn all aspects of research, from animal husbandry, to sample processing and data analysis. Through this approach I aim to foster collaboration and independence.
What big questions are you excited to explore next?

Some of the key questions I hope to answer about apple snail eye regeneration revolve around uncovering the fundamental biological mechanisms behind this remarkable process. One major area of interest is identifying the specific cell types responsible for regenerating all the eye components: the retina, lens, and cornea. Understanding whether these structures arise from a shared pool of cells or from distinct cell populations is essential to understanding how such complex tissues are rebuilt.
Equally important is exploring the genes involved in the regeneration process and how they are regulated. Dissecting these molecular circuits could reveal conserved pathways and highlight potential targets for biomedical applications.
Another critical question is how neural connections between the regenerated eye and the brain are re-established. While regenerating the physical structure of the eye is impressive, full functional recovery requires precise reintegration into the central nervous system. Studying how apple snails accomplish this could provide valuable insights into nervous system regeneration.
Finally, one of the most exciting prospects is the potential to identify specific genes or regulatory elements that can be tested in species lacking natural regenerative capacity. By comparing regenerative and non-regenerative systems, we may uncover key factors that could one day be harnessed to promote regeneration in humans.
Anything you’d like to highlight about your lab?
We are always interested in hearing from people who are excited about development, regeneration and snails and who would be interested in joining our team or collaborate with us! We highly value basic science, curiosity, creativity and community.
Now we have behind the science stories from Dr Alejandro Sánchez Alvarado !

Sánchez Alvarado Lab
Bluesky : @planaria1.bsky.social
X: @Planaria1
Image source: Stowers Institute for Medical Research
You’ve pioneered much of what we know about planarian regeneration. What motivated you to pivot toward the apple snail? What were your initial plans when you and Alice started the project?
Curiosity has always driven my research. After years delving into planarian regeneration, I wanted to take the lessons learned and test their validity in other systems. I knew from the work of Charles Bonnet (Observations sur la Physique, sur l’ Histoire Naturelle et sur les Arts, vol. 10, Paris, 1777, in Tracts on the Natural History of Animals and Vegetables, 2nd, ed., vol. II, Edinburgh, 1803, plate 8, p. 360) that some snails could regenerate their heads after decapitation. Given that such a head included complex sensory organs such as camera type eyes, I was intrigued to see how much regeneration was possible in snails and thought of it as a great opportunity to test how far fundamental principles of regeneration extend beyond our favorite models. When Alice and I initiated the project, we aimed to develop the apple snail into a powerful system, one where we could explore not only eye regeneration but new rules for organ complexity and repair.
Having studied planarians extensively, what similarities and differences strike you most between their eye regeneration and what you observed in Pomacea canaliculata?
In planarians, eye regeneration is fairly direct, that is, the structure is simple, and the set of participating cells is relatively constrained. Apple snail eyes, in contrast, are much more anatomically elaborate: they possess a lens, cornea, and a retina. Despite these differences, we observed the employment of a surprisingly conserved genetic toolkit, yet the deployment is tailored to the organism’s needs and eye architecture. While planarians offer lessons in simplicity and robustness, snails challenge us to understand regeneration in complex, multi-tissue architectures.
As you said, snail eyes are highly organized with a lens, cornea, and retina. How did you approach regeneration of a complex organ? What were your reactions when Alice and the team showed you the eye regeneration phenotype? How did you celebrate?
We approached snail eye regeneration with a mix of excitement and humility. Knowing the added complexity, our first step was to characterize the anatomy and developmental processes in exquisite detail, as we’d done in planarians. When Alice showed me the early phenotypes (eyes regrowing with partial or complete restoration of layers) it was exhilarating. There was a sense of witnessing something extraordinary, something no one had seriously documented in this way before. We asked ourselves: if this is the wild type (eye regeneration) imagine what phenotypes will we get once we can begin to genetically perturb this process? We celebrated in true lab fashion: with data, good coffee, and a shared sense of purpose.
For the broader scientific community, how important it is to move beyond conventional systems towards models which are more “problem suited”?

I believe science advances most meaningfully when we select models tailored to address questions, not just because they’re easy or fashionable. Apple snails forced us to reconsider mechanisms dogmatically ascribed to “higher” animals. For example, we unexpectedly found developmental modules acting outside canonical developmental windows, hinting at a flexibility in the animal’s response to injury or loss. Integrating these observations required both developmental and regenerative frameworks to be more plastic and open to revision. In essence, exploring unconventional systems not only expands our sense of what is possible in biology, but also reminds us, quite humbly, that we have yet to discover the full scope of what biology is already capable of achieving.
Across planarians, snails, and vertebrates, pax6 seems to act as a unifying thread in eye development. How do you see your work helping to connect these very different models into a broader evolutionary framework?
Pax6 is a beautiful example of deep homology: one gene at the crux of eye development in organisms as disparate as worms, snails, and humans. Our work allows us to chart the variations on a theme: the “melody” played by pax6, for example, shifts based on the “instrument.” This comparative approach helps trace evolutionary logic in how complex traits are built, lost, or re-invented, and fosters a more unified evolutionary understanding.
Was there a moment in this project that reminded you of your early planarian work—perhaps seeing the first signs of tissue re-emergence or recognizing a familiar gene playing a role in an unexpected context?
Absolutely. Seeing the initial re-emergence of eye tissue in snails, especially with familiar candidates like pax6 lighting up, evoked the earliest days in our planaria research. There’s a special thrill in spotting a familiar genetic face performing in a new “play.” These moments reinforce just how interconnected biology’s solutions really are. Perhaps more importantly, it presses us to recognize that, among countless possible outcomes, biology did not have to unfold in precisely this way, yet it did. The question, then, is why? What fundamental principles have shaped these solutions over evolutionary time, and might there be yet-undiscovered rules underlying these phenomena that the study of regeneration could help us uncover?
Do you imagine a comparative roadmap, linking regeneration in planarians, snails, and vertebrates, that might one day illuminate how regenerative capacity has been gained or lost across the tree of life?
One of my greatest hopes is for the field to embrace genuine comparative biology across multiple scales and levels of resolution—a comprehensive roadmap that interweaves regeneration in planarians, snails, vertebrates, and beyond. By charting where regenerative capacity is retained or lost, and probing the underlying reasons, we may finally decode the molecular signatures and constraints that shape these outcomes. This is an ambitious, long-term vision that traces its roots back to my earliest work (BioEssays, 22:578–590, 2000).
How do you look at processes of regeneration and development – where do they overlap, and where do they diverge?
Regeneration recapitulates development, sometimes literally, often figuratively. There are clear overlaps in gene regulatory networks and cell behaviors, but crucial divergences arise: injury response, aged tissue, functional integration of new tissues with old, and organismal context all shape outcomes. Examining both processes in parallel ensures our interpretations remain grounded and discerning, fostering an appreciation for both their commonalities and their distinctions.
You’ve mentored so many students and postdocs who have gone on to start their own labs and do incredible science. What is your mentoring philosophy?
Mentoring is, without question, the most rewarding aspect of this work. Science is inherently a human pursuit, and watching students and postdocs mature into independent thinkers is the ultimate measure of success. My approach centers on fostering autonomy, intellectual rigor, and genuine kindness. My greatest hope is that everyone who passes through my lab carries forward a deep sense of curiosity, confidence, and thoughtful skepticism wherever their careers take them. To me, choosing to mentor means embracing the responsibility to help cultivate scientists who will one day surpass us and, in doing so, move the field forward in ways we have yet to imagine.
Experiments don’t always work, and science can be frustrating. How do you help your students and trainees stay curious, motivated, and resilient during unfavorable circumstances?
I frequently remind my lab that failed experiments are the tuition we pay for discovery. I encourage tenacity by fostering a culture in which failures are shared, analyzed, and celebrated as learning opportunities. Curiosity is self-sustaining if it’s nurtured, and joy in small wins (finding a new phenotype, seeing cells behave unexpectedly) is kept front and center. It is important to emphasize that both true innovation and robust, lasting knowledge are built bit by bit, through careful testing, iterative refinement and the willingness to work patiently in the face of complexity, particularly when the prevailing winds conspire against such efforts. Our job as scientists is to contribute and continue to build a legacy of discovery that is as relevant tomorrow as it is today.
What do you find most awe-inspiring about nature’s capacity to regenerate, and how does that influence the way you think about biology?
To witness a fragment of an animal regenerate into a complex, living structure is to brush up against the truly profound. These moments evoke a sense of philosophical awe, as life reasserts itself with ancient, elegantly orchestrated mechanisms. Nature’s answers to damage and loss inspire both humility and an unshakable urge to understand how such feats are possible. In this light, every act of regeneration becomes a fresh retelling of an ancient narrative, one that has unfolded, again and again, across the history of life on Earth.
What continues to drive your curiosity and excitement about regeneration after all these years?
It’s the interplay of questions, the unexpected twists, and the pure delight in discovering something genuinely new. Regeneration is a frontier: every answer spawns new mysteries, and the joy of discovery, whether majestic or subtle, never fades.
A note from Shefali : I came across this beautiful research paper by Accorsi et al on bluesky and it literally blew my mind. It’s one of the rare times in the year when you stumble upon a piece of science that reminds you why you chose this path in the first place. As a grad student who is in the last leg of their PhD, it’s easy to to lose sight of the bigger picture – this paper brought it all back. I urge you all to read it—it’s rare and remarkable. Check out the Accorsi lab webpage and reach out if you’re interested in studying development and regeneration in snails.


 (10 votes)
(10 votes)